Relationship between water temperature and phytoplankton communities in Ba Lai river, Viet Nam
- Vietnam Academy of Science and Technology (VAST), Institute of Tropical Biology (ITB)
Abstract
Introduction: Phytoplankton which can affect higher trophic levels play a pivotal role as primary producers. Phytoplankton structure and diversity may, besides other factors, be controlled by the changing of water temperatures. Hence, the present study aimed to determine some relationship between phytoplankton assemblage and water temperature in Ba Lai River, Vietnam.
Methods: In this research, sample data along the Ba Lai Estuary during two surveys were carried out in rainy season (September, 2017) and in dry season (March, 2018), and analyzed by Spearman's correlation and Linear regression analysis to find the correlations.
Results: The results showed that the temperature of surface water in Ba Lai River was quite stable spatially. A total of 128 species belonging to 5 groups- namely cyanobacteria, diatoms, green algae, euglenids and dinoflagellates- were recorded in which diatoms were clearly dominant. Additionally, multiple stepwise linear regression revealed that phytoplankton assemblage correlated significantly with the temperature of surface water. The water temperature had a significant positive correlation with chlorophyll-a concentration but was negatively correlated with Margalef’s diversity index in the rainy season. Moreover, the significant negative association of water temperature with biomass of phytoplankton and biomass of diatoms, which was principal in species number, were determined in the dry season.
Conclusion: This study investigated the phytoplankton communities and found their correlation with environment in the area, and demonstrated advantages of phytoplankton which warrant their further research.
INTRODUCTION
Phytoplankton contribute to nearly half of the annual global primary production and are major drivers of biogeochemical cycling, thereby sustaining the food webs of most ecosystems; thus, they have garnered increased attention in research1. Phytoplankton are useful indicators of water quality because their spatial and temporal patterns reflect both short-and long-term environmental changes2. Therefore, changes in phytoplankton community structure and diversity can be evaluated to understand impact of environmental changes on ecosystems3.
The favorable environmental parameters ( pH, temperature, salinity, dissolved oxygen, and nutrients) in estuarine and coastal waters have been reported to affect and link with the diversity, distribution, abundance, and composition of phytoplankton4, 5. Temperature is among the major factor to influence phytoplankton growth rates, nutrient stoichiometry, and spatial and temporal distribution in freshwater systems. The alteration of seasonal temperature and increasing water temperatures, in addition to other parameters, may control phytoplankton growth and diversity. Additionally, biodiversity is decreasing at a rapid rate and this decrease is further accelerated by artificial climate change and associated rising temperatures on a global scale6. An increasing number of studies have focused on how phytoplankton primary productivity and species composition are influenced by water temperature, triggered by an interest to understand how global warming affects ecosystem processes and properties7. The combined effects of temperature and diversity on phytoplankton growth have been emphasized in recent study of Schabhüttl (2013)6. In this research, high temperature had a negative effect on phytoplankton diversity, in that a temperature-dependent decrease in diversity was most obvious in communities adapted to cooler base temperatures. Moreover, green algae and diatoms have shown a trend to perform better at lower temperatures, while cyanobacteria have shown a stronger relationship with increasing temperatures in mixed communities6. Besides, the main objective of the study in Tagus Estuary (Portugal) was to identify the key environmental factors affecting phytoplankton structure. BIOENV analysis revealed that in study period, the water temperature and other factors were the variables that had the strongest correlations with the phytoplankton community along the Tagus Estuary and best explained the phytoplankton spatial pattern8. The study in the Yangtze River (China) observed that water temperature, nutrient concentrations and light availability were the driving factors that determine phytoplankton dynamics9.
Ba Lai River is a river in the Mekong Delta region which flows through Ben Tre Province. It is a direct tributary of Tien River and the natural boundary between Phu Duc and Tan Phu Communes. At the beginning of the 20th century, the river became alluvial, narrow and shallow. Nowadays, the water source of Ba Lai River comes mainly from the My Tho River. Ba Lai River has also begun to diminish while Ba Lai Estuary is filling with sediment and becoming blocked. In 2002, in the Ba Lai Estuary, the construction of the Ba Lai dam which functions to prevent salinity, create a fresh source of water, and supply water for the districts of Binh Dai, Ba Tri, Giong Trom, Chau Thanh in Ben Tre Town, has been considered as an environmental failure for the originally connected estuarine and river ecosystems10. Several studies have warned that the Ba Lai dam could result in a high disturbance and deposition of this river11. According to a recent study of phytoplankton community structure in Ba Lai River, it was revealed that the water quality had shown signs of serious deterioration through biological indicators and there were other evidence which indicated that the Ba Lai dam negatively affected the phytoplankton communities. The phytoplankton distribution was influenced by the environmental parameters 12.
The main objectives of this paper were to characterize the influence of water temperature, one of the main environmental factors, on controlling the distribution pattern of phytoplankton communities and diversity in the Ba Lai River, therefore contributing to the assessment and monitoring of aquatic environments.
MATERIALS AND METHODS
Study area and field sampling
Two surveys were conducted at 10 sites (represented by nomenclature BL1 to BL10) in the Ba Lai River in March of 2018 (dry season) and September of 2017 (rainy season) (
Locations of sample sites
| Sites | Sampling coordinates | |
| Latitude | Longitude | |
| BL1 | 10°17'29.8"N | 106°12'40.2"E |
| BL2 | 10°18'43.7"N | 106°17'38.5"E |
| BL3 | 10°17'38.8"N | 106°21'22.0"E |
| BL4 | 10°16'10.8"N | 106°26'24.5"E |
| BL5 | 10°12'30.6"N | 106°32'6.48"E |
| BL6 | 10°11'05.8"N | 106°34'35.8"E |
| BL7 | 10°10'17.7"N | 106°36'48.6"E |
| BL8 | 10°08'44.9"N | 106°38'01.1"E |
| BL9 | 10°08'17.1"N | 106°38'35.6"E |
| BL10 | 10°02'38.5"N | 106°41'03.7"E |

Sampling stations in Ba Lai river, Ben Tre province.
Planktonic diatom samples were collected from the surface waters by towing a conical net made of bolting silk with 25 μm mesh size. Subsequently, samples were kept in 150 ml plastic bottles, preserved in 4% neutralized formalin and used for qualitative analysis; chlorophyll-a analysis samples were collected by used plastic cans (each with a capacity of 2 L of surface water), and preserved in 4% neutralized formalin for qualitative analysis13.
Planktonic diatom identification and chlorophyll-a analysis
Samples were examined with an inverted microscope (CK40, Olympus, Japan) at 200× or 400×magnification. Identification was based on morphology per examples from the literature14, 15, 16. The classification of phytoplankton into taxonomic groups and verification of currently accepted taxonomic names followed AlgaeBase17. At least 500 cells were counted under Sedgewick counting technique, a method by Sournia (1978) to determine abundance18. The biomass of cells was calculated based on geometrical formulas according to Sun and Liu (2003)19. Biovolume was calculated based on geometrical cells or colony volumes and subsequently converted to biomass (wet weight) by assuming a specific gravity of 1 mg/mm (Wetzel and Likens, 2013)20.
In order to analyze chlorophyll-a concentration, about 100-300 mL samples were filtered through GF/C filter paper. The filter was subsequently frozen until sample processing. Chlorophyll-a was dissociated with 90% acetone solution overnight at room temperature and in the dark. The samples were centrifuged at 400 rpm for 20 minutes to discard scum. Chlorophyll-a in the extract solution was analyzed by an UV-DR-500 spectrophotometer (Hach, USA).
Data analysis
The phytoplankton community structure of species richness was assessed by Margalef’s index (d) and Pielou’s evenness index (J’) which were used to characterize the phytoplankton community at each site. These metrics were calculated by using the PRIMER VI analytical package developed by Plymouth Marine Laboratory (U.K.).
One-way analysis of variance (ANOVA) was used to test the significance of the differences among the sites based on the water temperature and the phytoplankton species structure metrics. The analysis was completed, using Tukey's HSD test significant difference. The correlations between the water temperature and the phytoplankton community structure and diversity were determined by Spearman's correlation analysis method, and then linear regression analysis was performed. All variables were log-transformed (log + 1) to normalize their distributions before analysis. Statistical calculations were performed using Statgraphic centurion XV.
RESULTS
The water temperature and chlorophyll-a concentration
Temperature is important because it has a massive effect on determining what organisms can survive in a body of water and also directly affects the rate of photosynthesis and sensitivity of organisms’ toxic wastes21. The results showed that temperature of surface water in the Ba Lai River was quite stable spatially. It varied between a minimum rate of 27.60°C in the dry season and a maximum rate of 33.95°C in the rainy season (Figure 2). One-way ANOVA was performed and showed that no significant difference in the surface water temperature was detected between the two seasons (p>0.05). The temperature of the survey in Ba Lai River was relatively stable and there were not so large fluctuations between the sample sites and between the two surveys. Ba Lai River is located in the tropical region which has a similar water temperature as some other bodies of water in Southern Viet Nam (range of 28–32°C)22. The average water temperature of approximately 30°C reflects positive conditions for development of phytoplankton23.
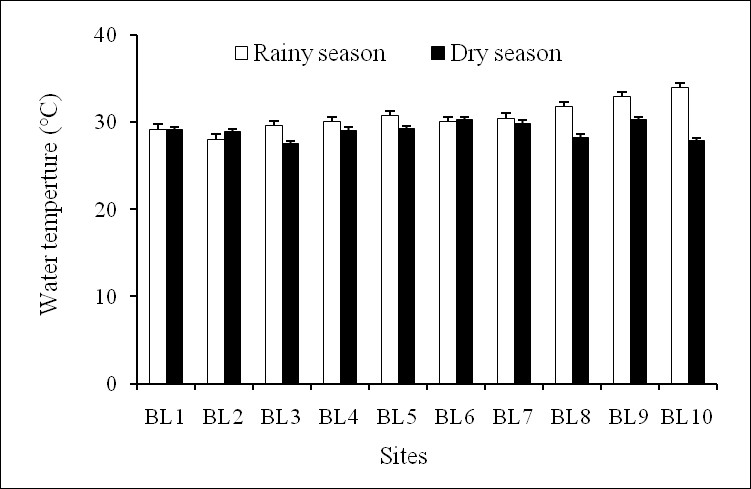
The water temperature from sampling sites in dry and rainy seasons.
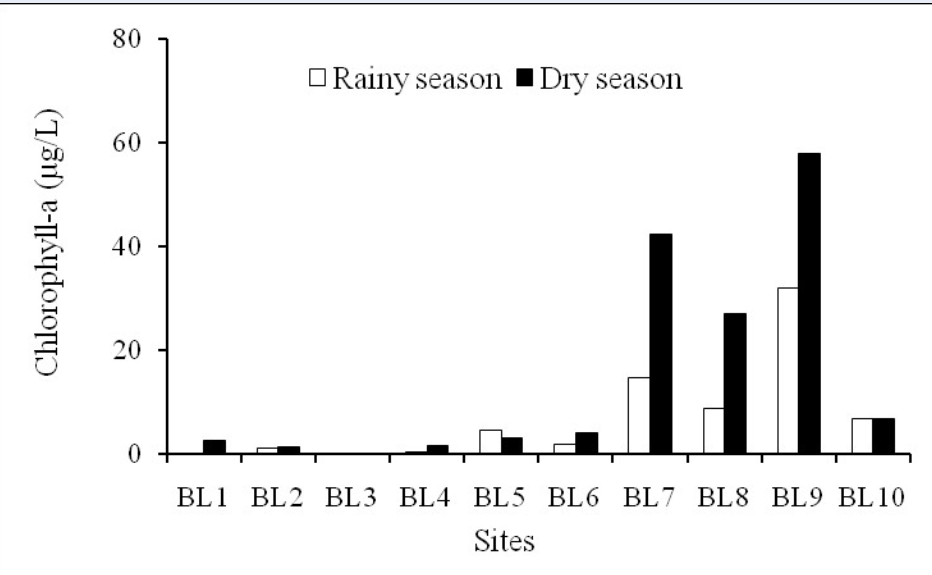
Chlorophyll-a concentration varied between 0–58.05 µg/L with the minimum and maximum concentrations occurring in the dry season (Figure 3); these concentrations are higher in some seaward areas than in the inner zone of the Ba Lai River estuary. There was a significant increase from the upstream sections to the downstream river sections in both surveyed seasons. Generally, chlorophyll-a concentration was high at stations BL7, BL8, and BL9; it was highest at BL9 station (which was below the Ba Lai dam). Meanwhile, BL3 station had the lowest chlorophyll-a concentration. Phytoplankton need light and nutrients to develop. Since the water environment of Ba Lai River had high alluvial content, turbidity and strong disturbance, these factors prevented light from entering the water environment, leading to poor growth and development of phytoplankton, and resulting in low chlorophyll-a content in that area. BL3 station had high turbidity and total suspended solids (TSS) values that led to low concentration of chlorophyll-a.

Chlorophyll-a concentration in two seasons.
Phytoplankton composition and abundance
Species composition of phytoplankton
From the two surveys, a total of 128 phytoplankton species, belonging to 5 phyla and 76 genera were identified from the Ba Lai River (Figure 4). The phytoplankton species were predominantly (69 species, gaining 53.91% of total species number), followed by (25 species, 19.53%), (19 species, 14.84%), (8 species, 6.25%), and (7 species, 5.47%) (Figure 4C). The number of was most dominant in both seasons which could be related to the fact that diatoms could thrive well in varying environmental changes, while the number of and were lower than the other phylas. The species number of phytoplankton in the rainy season was higher than that in the dry season. Particularly, the number of was only recorded in the lower part of the Ba Lai dam in the dry season, while the number of and often appeared at sites near the upper part of the dam in the rainy season. This corresponded to the study conducted on the Ba Lai River by Pham (2017)12.
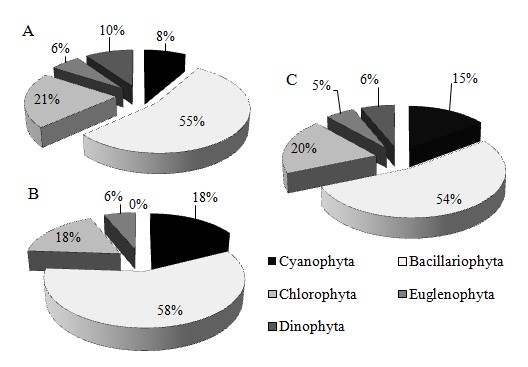
The seasonal distributions of phytoplankton composition in Ba Lai River in dry season (A), rainy season (B), and both seasons (C).
Phytoplankton abundance and biomass
Phytoplankton densities ranged from 301–1185 × 10cells/L in the rainy season and from 303–683 × 10cells/L in the dry one. The maximum abundance (up to 1185 × 10 cells/L) occurred at BL9 station in the rainy season, whereas the minimum density (301 × 10 cells/L) was recorded at BL5 station in the rainy season (Figure 5). The BL9 station had highest density because of the increasing abundance of several cyanobacterial species, including and ,accompanied by the decreasing abundance of marine diatoms. The phytoplankton usually distributed according to the rule that the freshwater species (such as cyanobacteria and green algae) were more observed at upper stations and decreased at lower stations, while the phytoplankton communities were more characterized by marine diatoms in downstream stations. However, at the sites near the Ba Lai dam, the distribution of phytoplankton communities was disturbing. At stations BL8 and BL9 (which are considered downstream stations), the abundance of cyanobacteria and green algae were higher than marine diatoms. Besides, cyanobacteria has an advantageous characteristic which is optimal growth at higher temperatures, whereas diatoms grow better at relatively lower temperatures22, 24. The phytoplankton species which were predominant in quantitative samples at some sites of the Ba Lai River were as follows: , , , . At BL6 station in the rainy season, the species of Euglenophyceae densities that were dominant were and,which revealed the mesotrophic to eutrophic conditions of the body of water25.
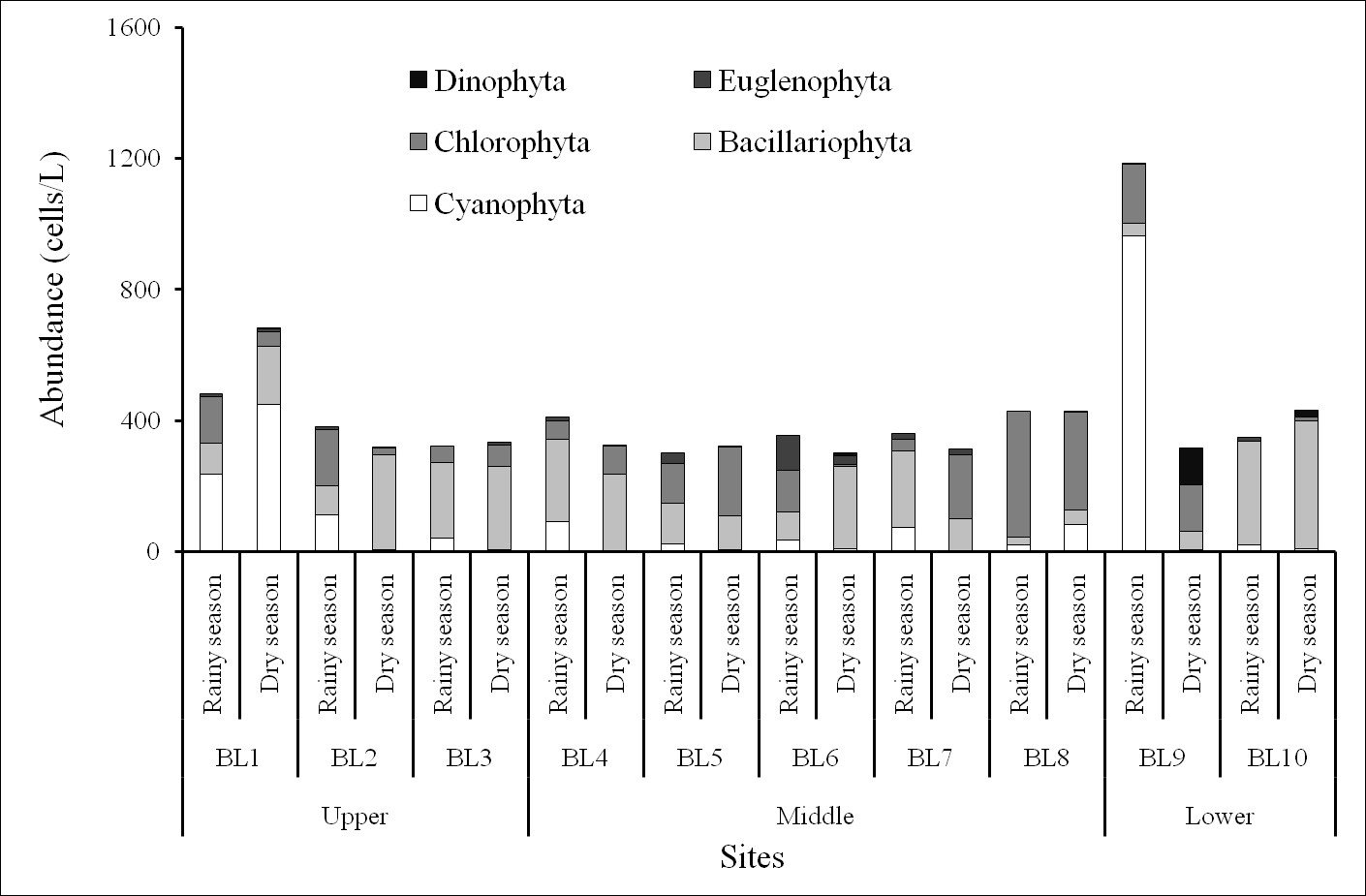
The abundance of phytoplankton in the Ba Lai River.
The biomass varied from 5.27–17.09 µg/L in the dry season and from 3.83–27.31 µg/L in the rainy season (Figure 6). The maximum and minimum biomasses occurred at BL3 and BL9 stations, respectively, in the rainy season. Biomass of was dominant at most sites, which represented the greatest contribution to phytoplankton biomass in the Ba Lai River. The restriction of light entering the environment led to poor growth and development of microalgae because of rich alluvial deposits, which was one of the reasons for the low density and primary biomass of phytoplankton in the shallow and turbid estuary systems. Corresponding to the abundance of phytoplankton, the biomass of cyanobacteria and green algae significant increased at the sites near the Ba Lai dam (where there was actually a marked succession of diatom species) along with the high salinity gradient. The biomass of was high at the other sites. Moving to the upstream sites, the high abundance and biomass of diatoms that adapted to the saline environment were more distributed. This could explain the marine species being carried by the saline flows from the tributaries of the Ba Lai River12. This could be a disturbance which greatly explains why the phytoplankton communities were probably influenced by the dam.
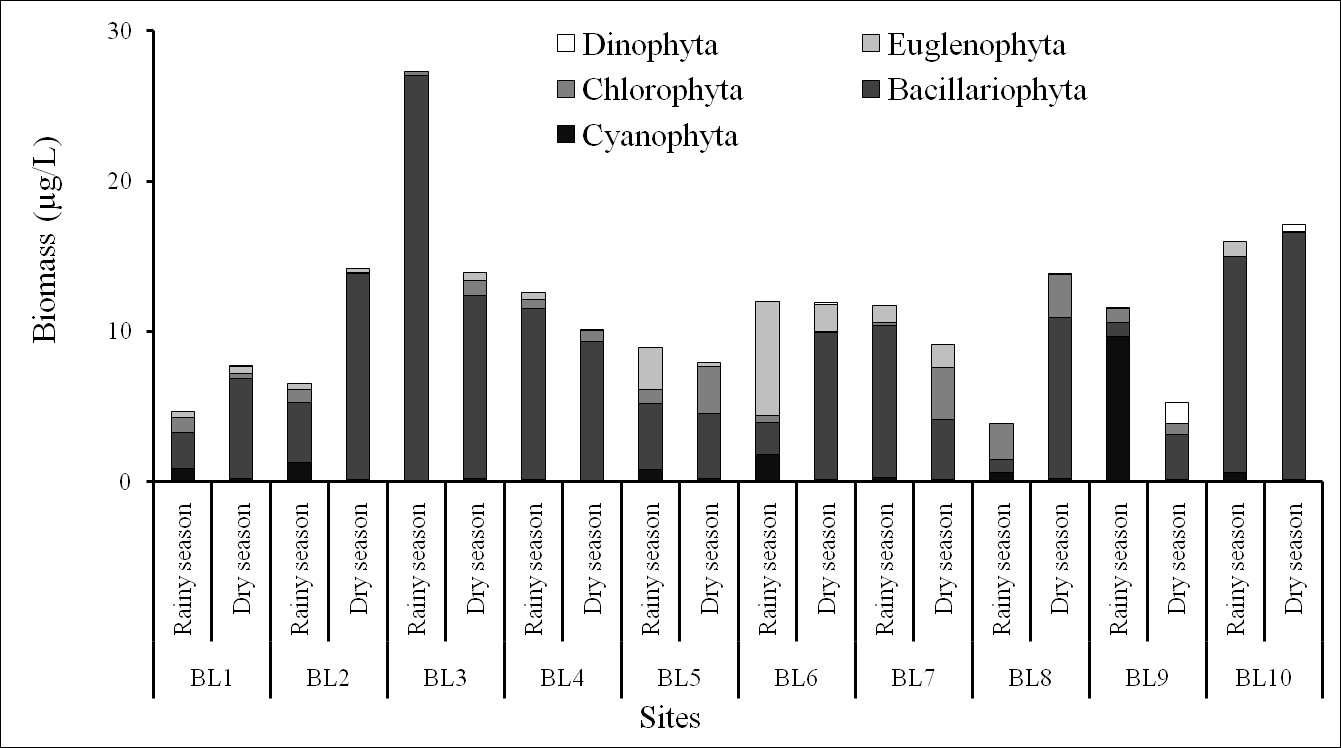
The biomass of phytoplankton in the Ba Lai River.
Biological indices of phytoplankton community
Temporal and spatial variations of phytoplankton metrics are shown in Figure 7.Margalef’s diversity index (d) measured density of species in an ecosystem. The higher the index value, the greater the species richness over the study area, and vice versa26. Margalef’s diversity index (d) ranged from 2.31–6.56 and 2.60–5.36 in the dry and rainy seasons, respectively. The maximum and minimum biomasses occurred in the BL2 and BL8 stations, respectively, in the rainy season. One-way ANOVA was performed and no significant difference of the d index was detected between the two seasons (p>0.05). The diversity at the sites was relatively high and was evenly increased from the dry season to the rainy season, except for stations BL8 and BL10, of which the dry season is higher than the rainy season. The number of species in both sites was higher in the dry season than in the rainy season, whereas the rest was opposite. Evenness index (J’) showed the stability of the phytoplankton communities in the ecosystem, throughout this indicator showed the balance of the community26. Evenness index (J’) was also high, and 80% of values surpassed 0.7. The index ranged from 0.62 to 0.89 and from 0.46 to 0.81 in rainy and dry seasons, respectively, and no significant difference of J’ index was detected between the two seasons (p>0.05). The BL8 station was lower than the other stations which indicated that the phytoplankton community was dominated by a few species coinciding with the observation of the blooms of in the study area during both seasons.

Phytoplankton metrics (d, J’) in the Ba Lai River
Relation of the water temperature to the phytoplankton communities’ structure and biodiversity
The correlation between the water temperature and the phytoplankton communities’ structure and bio-diversity were evaluated by Spearman's correlation analysis and linear regression analysis. The results of the Spearman's correlation analysis between the water temperature and phytoplankton communities’ structure and biodiversity in the rainy season are shown in
The correlation coefficient between the water temperature and phytoplankton communities structure, biodiversity in both seasons
| Variables | The water temperature | ||
| Rainy season | Dry season | ||
| Abundance of phytoplankton | r | -0.024 | -0.711 |
| p-value | 0.942 | 0.033* | |
| Abundance of Bacillariophyta | r | 0.037 | -0.462 |
| p-value | 0.913 | 0.166 | |
| Biomass of phytoplankton | r | 0.11 | -0.742 |
| p-value | 0.743 | 0.026* | |
| Biomass of Bacillariophyta | r | -0.529 | -0.790 |
| p-value | 0.112 | 0.018* | |
| Chlorophyll-a concentration | r | 0.793 | 0.376 |
| p-value | 0.017* | 0.259 | |
| d index | r | -0.711 | -0.363 |
| p-value | 0.033* | 0.276 | |
| J’ index | r | 0.103 | 0.007 |
| p-value | 0.757 | 0.985 | |
Additionally, the linear regression analysis indicated the relationship between the water temperature and chlorophyll-a concentration, and d index, as illustrated in Figure 8. The water temperature had a positive correlation with chlorophyll-a concentration, using a relatively low adjusted coefficient of simple linear regression model (adjusted R=43.787%, p=0.022). Meanwhile, the water temperature was significantly and negatively correlated with d Index and a substantially predictive capability (R=49.599%, p=0.014).

Model of linear regression analysis in the rainy season. A. The correlation between water temperature and chlorophyll-a concentration, B. The correlation between water temperature and d Index.
Furthermore, the results of stepwise multiple linear regression analysis showed that when combining the concentration of chlorophyll-a and the d Index, the relationship between water temperature and the two factors could be interpreted in a highly significant model. The observed versus predicted water temperatures in the Ba Lai River are shown in Figure 9. That is, the water temperature positively correlated with concentration of chlorophyll-a but had a negative relationship with d Index.
Water temperature = 1.5602 + 0.0232414 × Chlorophyll-a - 0.103903 × d Index
(with: R = 71.74%, p=0.005)

The observed versus predicted water temperatures in the Ba Lai River.
The results of the Spearman's correlation analysis on water temperature and phytoplankton communities’ structure, and biodiversity in the dry season indicated that the water temperature negatively correlated with abundance of phytoplankton, biomass of phytoplankton and biomass of (

Model of Linear regression analysis in dry season. A. The correlation between water temperature and biomass, B. The correlation between water temperature and biomass of Bacillariophyta.
DISCUSSION
Phytoplankton reflect and affect water quality which changes in its community structure, patterns of distribution and the proportion of sensitive species. In our study, the phytoplankton assemblages in the Ba Lai River included freshwater, estuarine and freshwater, and estuarine and marine species (which were dominated by diatoms). The dominance of this group (diatoms) has been already reported by other authors either along the river or estuary; however, studying in running water conditions are more appropriate for evaluating growth of diatoms8, 27, 28. Generally, dominance of diatoms indicates the physical inconsistency of the shallow coastal environments which are very common to study; most diatoms are robust and remain in the estuarine environments in spite of the erratic salinities29, 30.
Biodiversity is an important component of ecosystem functioning and stability and it has been widely used to characterize community structure. The diversity of a community depends on the Species Richness and Species Evenness31, 32. The Margalef’s Diversity Index and Evenness Index were relatively high, which explains that the evenness between the survey sites reflects the uniformity and stability of ecological characteristics in the area. However, there were many clear evidences that the Ba Lai dam caused many negative effects on phytoplankton community and biodiversity in the river.
The environmental parameters influence the phytoplankton distribution; of these parameters, water temperature plays a fundamental role and induces marked changes in a community structure. In the rainy season, the water temperature had a positive relationship with chlorophyll-a concentration but negative correlation with d Index. That means if the water temperature increased, chlorophyll-a content increased. However, while there was an increase in water temperature, the d Index decreased, and vice versa. Chlorophyll-a concentration is a convenient index of phytoplankton biomass, and water temperature is an important factor which influences the control of the growth and distribution of phytoplankton32, 33, 34.
In the aquatic ecosystem, chlorophyll-a concentration usually is recognized as a surrogate for biomass of phytoplankton which is dependent primarily on several environmental parameters and has been studied in recent studies35. According to the study in Meiliang Bay (China), the concentration of chlorophyll-a was significantly correlated with water temperature; a multiple stepwise linear regression explained 99.2% of the variation of chlorophyll-a. However, in Taihu Lake (China), the water temperature explained 98.7% of the variation of chlorophyll-a.
Thus, water temperature is an important factor influencing the annual change of chlorophyll-a concentration and phytoplankton biomass36. In general, biodiversity is an important factor which determines phytoplankton community performance under varying temperature conditions6. Biodiversity is a useful alternative to reflect the ecological quality of aquatic ecosystems because biological communities are influenced and integrated by the environmental effects of water37. The warmth of the water temperature and environmental instability caused changes in phytoplankton diversity and community structure, which are indispensable characteristics underlying ecosystem functioning and trophic transfer38. From the research in Vam Co River (Vietnam), the phytoplankton biodiversity positively correlated with water temperature and species number39. Moreover, when combining the concentrations of chlorophyll-a and d Index, the highly significant model of water temperature was predicted (Figure 9). Increasing the concentration of chlorophyll-a will lead to rise of water temperature but it is limited by the diversity index. Likewise, in the dry season, the water temperature had a significant negative correlation with biomass and the biomass of found in this study. That means, if the water temperature increased, the biomass of phytoplankton and biomass of decreased, and vice versa. The impact of temperature on phytoplankton entails nutrient uptake, death rate, and nutrient release from particulate nutrients, abundance, and biomass; all of these parameters were investigated by recent studies. The effects of seasonal temperature and daily temperature on phytoplankton biomass were simulated numerically and showed that the phytoplankton biomass was strong to the effects (and variation) of water temperatures, according to the dynamics of the model and model predictions in Lake Tai (China)38. Changes in phytoplankton biomass and photosynthesis in relation to temperature were showed in the Western English Channel40. Dinoflagellates and biomass showed a positive correlation with respect to temperature, reaching the highest biomass (between 15°C and 17°C). On the other hand, diatoms indicated a negative correlation with temperature, with highest biomass at 10°C41.
Besides the other abiotic variables, the variability of water temperature can be considered a major factor contributing to the changes of the phytoplankton community structure, which was favorable for the development of chlorophyll-a concentration, diversity index and biomass. According to the studies in the Ba Lai River, there were more factors influencing phytoplankton growth. For instance, the phytoplankton assemblage was affected by total dissolved solids, salinity and nutrients. This indicates that in addition to nutrient concentrations, the total dissolved solids significantly influence the phytoplankton community structure as a result of high turbidity affected by tides and silt accretion in the estuary12. Thus, predictive models for relationships between water temperature and phytoplankton community structure are preliminary data which can provide baseline information and be used as critical ecological tools for water quality management.
CONCLUSION
Results of this study contributed the advantageous information on phytoplankton structure and diversity, as well as their correlation with water temperature in running water systems which are becoming more and more popular in Vietnam. The phytoplankton assemblage in the Ba Lai River was typical in the shallow and turbid estuarine systems where diatoms were clearly dominant in both the dry and rainy seasons. Additionally, the results illustrated that the temperatures of surface water from the Ba Lai River did not vary among the sampling sites and was similar to the water temperature of some other rivers in Southern Vietnam, which were beneficial for the development of phytoplankton. The relationships showed that rises in water temperature could significantly elevate the chlorophyll-a concentration but decrease the diversity index, biomass of phytoplankton, and biomass of diatoms (which were the primary species in terms of species number). Water temperature is one of the key factors that changes phytoplankton distribution and associates with phytoplankton bloom, in turn influencing the whole food chain and which can seriously impact future global warming. Therefore, it is essential to observe how the changes of environmental parameters impact phytoplankton assemblages in river ecosystems so that the water supply issues in the region can be effectively managed.
COMPETING INTERESTS
The authors declare that there is no conflict of interest regarding the publication of this article.
AUTHORS’ CONTRIBUTIONS
The contributions of all authors are equal in selecting data, calculating descriptors, analyzing results, and writing a manuscript.
ACKNOWLEDGMENTS
This research was funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.06-2019.51.

