New method for preparing purity β-D-glucans (beta-Glucan) from baker’s yeast (Saccharomyces cerevisiae)
- Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology
- Nhatrang Institute of Technology Research and Application (NITRA), Vietnam Academy of Science and Technology (VAST), Nha Trang, Vietnam
- Institute of Natural Products Chemistry (INPC), Vietnam Academy of Science and Technology (VAST), Cau Giay, Hanoi, Vietnam
Abstract
Introduction: β-D-glucans (beta-Glucan), a water-soluble polysaccharide with diversity physiological activities for applications in food and pharmaceutical industries.
Methods: In this paper, we report the use of ionic liquid 1-butyl-3-methyl-imidazolium chloride [BMIM]Cl on the extraction and isolation of β-glucan from baker's yeast Saccharomyces cerevisiae. The β-D-glucans precipitated by adding water into the solution and obtained by filtration or centrifugation were pure, cleaned, and free of cell membranes.
Results: The beta-glucan was obtained as white precipitates after adding water into the mixed solution. The 1D and 2D-NMR spectrum and titration methods applied for qualitative and quantitative determination showed that the beta-glucan product contained 78.2% 1,3-β-D glucan with 98.4% purity.
Conclusion: This method can be used to prepare purified beta-glucan from baker’s yeast.
Introduction
-D-glucans (beta-glucan) are polysaccharide consisting of glucose molecules, linked by -(1-3) and/or -(1-6) linkages (IUPAC Recommendations 1995) with physiological diversity activities for applications in food and pharmaceutical industries 1. It is well-known to be the strongest natural immune-enhancing compound 2. -D-glucans are anti-carcinogenic agents through the activation of macrophages, T-cells, and NK cells for defending the immune system 3. -D-glucans are found in the cell walls of many microorganisms, including plants, such as oats and barley, bacteria, fungi, algae, lichens, and yeast 4. The cell wall of yeast is one of the important b-glucan source 5.
The extremely low solubility of yeast -d-glucan in water due to the relatively strong intermolecular hydrogen bonds between hydroxyl groups of the glucose units in the -D-glucan chains causes these products difficult to extract from yeast and therefore limits its application 5. Thus, using ionic liquid composed of full of cations and anions, high polarity, and low-melting-point salts are able to increase the solubility of yeast -D-glucan and consequently increase the yield of b-glucan extracted from yeast 5. In order to obtain -D-glucan with high yield and high purity for application as a pharmaceutical product, in this paper, we described method using the ionic solution to produce beta-glucan from baker's yeast (). The -D-glucans precipitated by adding water into the solution and obtained by filtration or centrifugation were pure, cleaned, and free of cell membranes.
Materials and Methods
Beta-D-glucan 98%, 1-chlorobutane, 1-methylimidazole, KFe(CN), NaOH, HSO were purchased from Sigma-Aldrich (USA) and used as obtained. Acetonitrile and ethyl acetate (Merck) were distilled over phosphorus pentoxide (PO) and stored over molecular sieve 4A before use. 1D and 2D-NMR spectra were measured on a Bruker AVANCE 500 spectrometer in deuterated solvents as DMSO – , CDCOOD- or DO-d (Sigma-Aldrich (USA)).
Preparation of ionic solution [BMIM]Cl
The ionic solution 1-butyl-3-methyl-imidazolium chloride [BMIM]Cl was prepared according to the method of Dupont et al. 6. Briefly, 150 g 1-methylimidazole, 80 mL acetonitrile, and 220 g 1-chlorobutane were added in a 2-L, three-necked, round-bottomed flask and refluxed at 80 C in a heating oil bath for 48h. The excessive volatile material was removed by vacuum distillation. The remaining light-yellow oil was re-dissolved in dry acetonitrile (250 mL), and the solution was dropwise added into a well-stirred solution of dry ethyl acetate (1000 mL). One seed crystal of 1-butyl-3-methylimidazolium chloride was added, and the flask was cooled at −30°C for 2 hr to initialize the exothermic crystallization process of [BMIM]Cl. Remove the supernatant solution through a filter cannula under pressure buildup from dry nitrogen. Dry the remaining white solid under reduced pressure (0.1 mbar, 0.001 mm) at 30°C overnight to afford [BMIM]Cl 282.5 g (~87%), mp 65-67°C. The products were identified using H-NMR spectrometer and compared the spectroscopic data with those in published literature.
Extraction of beta-glucan from baker's yeast ()
5g dry baker's yeast powder Saf-Viet® was added in 100g [BMIM]Cl in a 500ml glass beaker. Stirred the solution in 30 min at 80 C until all powder was solved in [BMIM]Cl in order to obtain a clear solution. Added 200 ml water and stirred for another 15 min. Crude beta-glucan was separated as precipitates, filtered and washed 3 times with hot water to remove impurity substances and dried overnight at 50 C to obtain pure beta-glucan. 125 g -D-glucan was obtained from 1kg dried yeast, so yield ~ 83.5% (compared to beta-glucan originally contained in the yeast).
Determination of -D-glucan content in the product
An accurate weigh of 0.100 g of the standard -glucan (Sigma-Aldrich), the baker's yeast, and beta-glucan product were dissolved in 2 ml ice-cold KOH 2 M in three tightly screwed 15 ml centrifuge tubes. Vortex for 20 minutes to dissolve all impurities as α-glucan, mannoprotein, glycogen. Centrifugation and remove the supernatant. The precipitate was washed with 2 ml ice-cold distilled water (2x). Centrifuge and remove the washing solution.
The samples were then hydrolyzed with 2.0 ml ice-cold HSO 12M solution. Vortex well in ice for 10s. Add 10 ml distilled water and incubate at 100 C for 2h. The solutions were then cooled to RT, filtered to remove impurities, and slowly neutralized with NaOH 5% in the presence of 3 drops of methyl red until a yellowish appeared. Add water to 100ml in volumetric flask and mix (Solution A). Add 10ml of KFe(CN) 1% solution and 2.5ml NaOH 2.5N in a flask, (boil the mixture) and titrate with sample solution A containing reducing sugar from the burette. The initial solution had a lemon-yellow color of potassium ferrocyanide. The titration stops were determined when the lemon yellow disappeared, the solution became colorless or transparent for about 30 seconds and then turned to the very pale straw yellow color of ferricyanide, consuming V (ml) of the sample solution. Do the same procedure with baker's yeast and beta-glucan product samples, consuming V and V. The reaction occurred as shown as following 7
V, V and V (ml) were the volumes of hydrolyzed -D-Glucan needed to reduce Fe in KFe(CN) to Fe of the standard (98%, Sigma-Aldrich), baker's yeast and beta-glucan product samples, respectively. The average volumes of 3 times titrations were used for calculation according to formulas:
The glucan amount was presented in the baker's yeast P = 0.98 x V/V.
The amount of beta-glucan was presented in the products P = 0.98 x V/V.
Results
The ionic liquid [BMIM]Cl
The obtained synthetic ionic liquid was examined by H-NMR spectrum (Figure 1).

1H-NMR spectrum (D2O) of the ionic synthetic liquid 1-butyl-3-methyl-imidazolium chloride [BMIM]Cl.
In the spectrum showed the presence of proton signal of one methyl group (-CH) at (ppm) 0.84 (3H, t, H-4') and three successive methylene groups (-CH-) belonging to a butyl group at (ppm) 1.24 (2H, m, H-3'), 1.78 (2H, m, H-2') and at (ppm) 4.12 (2H, t, H-1'). In addition, the spectrum also showed the presence of three typical –CH- signals of one imidazole ring at (ppm) 7.36 (1H, s, H-5), 7.41 (1H, s, H-4) and 8.65 (1H, s, H-2), and finally, the proton of a methyl group associated with an electrophile center of the ring at (ppm) 3.82 (3H, s, H-1'') 6. Compare to the literature; it can be established that the obtained product was 1-butyl-3-methyl-imidazolium chloride [BMIM]Cl 6. This compound was then used to prepare ionic liquid to extract beta-glucan from the baker's yeast.
Beta-glucan product
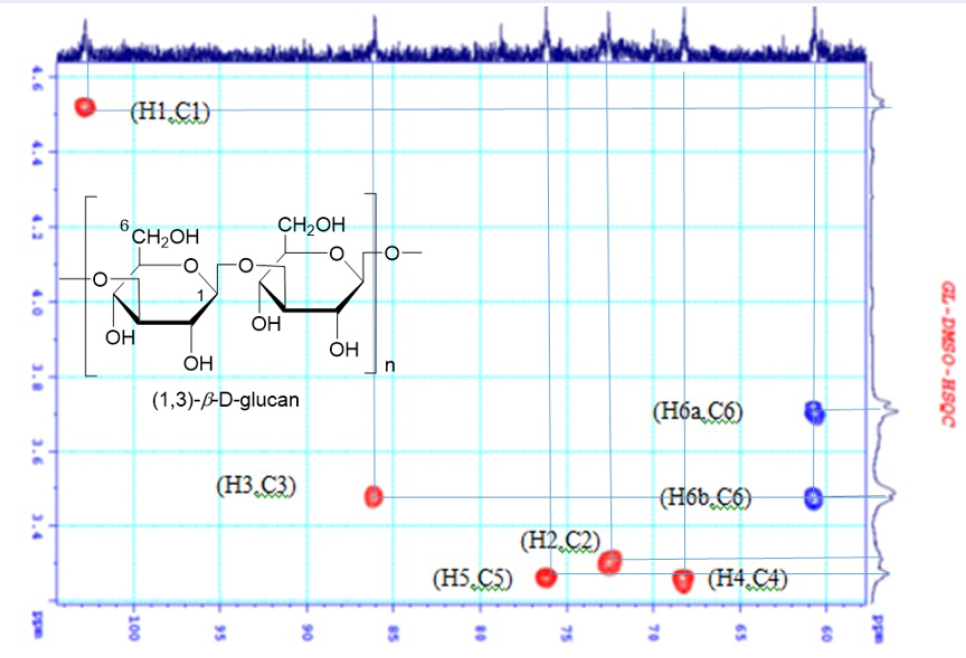
The beta-glucan extracted from baker's yeast () using the synthetic ionic liquid [BMIM]Cl was checked qualitatively by 1D- and 2D-NMR spectrum. HSQC spectrum of the product (Figure 2), showing the cross-peak of carbon signals at d (ppm) 103.4 (C-1), 70.1 (C-2), 85.7 (C-3), 69.1 (C-4), 76.8 (C-5), and 61.9 (C-6) of 1,3--D-glucan, to corresponding protons at (ppm) 5.15 (H-1), 4.0 (H-2), 4.2 (H-3), 3.8 (H-4), 3.6 (H-5), 4.3 (H-6) and 4.1 (H-6'), respectively 8. The water signal appeared at 4.7. ppm 9 (Figure 2).
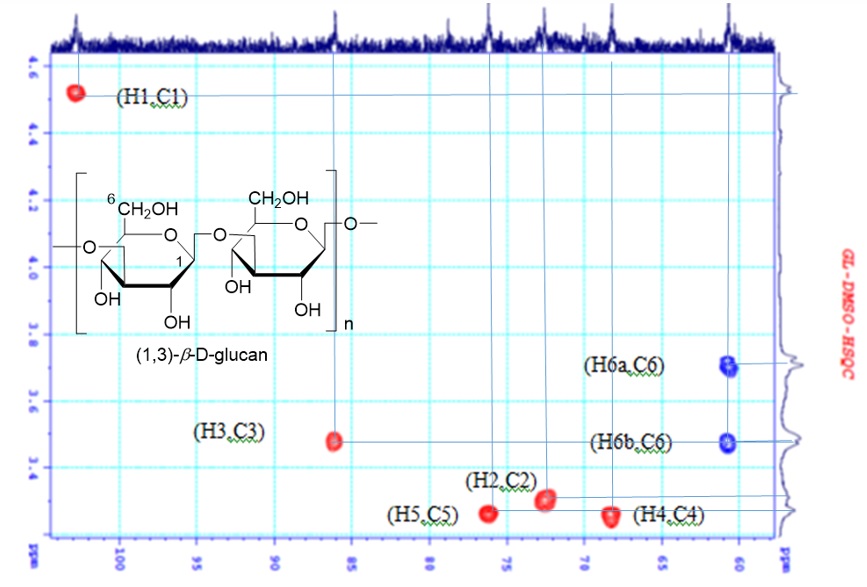
HSQC spectrum (DMSO-d6) of the obtained product 1,3-beta-D-glucan.
Cell-wall glucans are mainly composed of 1,3--D-glucan, mannan (a common carbohydrate in baker yeast), and 1,6--D-glucan.
Compare to the paper published by Ohno et al. 8 and Gonzalez et al. 10, whose -glucan product had a proton peak of water trace at ~ 4.2 ppm and mannan signal at > 4.9 ppm (in DO-). Calibrated the water peak of our measurement spectrum in CDCOOD- from 4.7 ppm to ~ 4.2 ppm, then no signals belonging to mannan carbohydrate impurity at 4.9 – 5.5 ppm were found, indicating that our -glucan product was clear from mannoprotein. However, the product contains 1,6--D-glucan with the proton signal of H-1 at ~ 4.9 – 5.0 ppm (Figure 3). Thoroughly, the H-NMR spectrum showed that signals attributable to the 1,3--D-glucan were mostly observed, while those of the mannan and the 1,6--D-glucan were hardly visible (Figure 3).
Based on peak integration in our H-NMR spectrum, the proportion ratio of this 1,6beta-glucan / 1,3beta-glucan was 0.186 : 1.000 (1.302 : 7.000) 15.6% / 84.4% (Figure 3). These facts suggested that the extraction by ionic liquid was rather selectively to obtain 1,3--D-glucan.

1H-NMR spectrum (CD3COOD-
Determination of glucan content in the product
The glucan content in the obtained product was determined using titration method with KFe(CN) in alkaline. The titration was conducted three times. The titration results were presented in
Determination of glucan content in the product beta-glucan
| Volume of hydrolyzed beta-glucan (ml) | 1st (ml) | 2nd (ml) | 3rd (ml) | Average (ml) | % b-glucan |
| V0 (standard sample) | 10.10 | 10.10 | 10.10 | 10.10 | |
| V1 (yeast sample) | 69.10 | 69.30 | 69.50 | 68.97 | P1= 0.98 * 10.1 / 68.97 = 14.3% |
| V2 (product sample) | 10.50 | 10.40 | 10.40 | 10.43 | P2= 0.98 * 10.1 / 10.43 = 95.2% |
Discussion
The ionic liquid [BMIM]Cl was synthesized from 1-chlorobutane and 1-methylimidazole in acetonitrile, according to Dupont et al. 6. The obtained synthetic ionic liquid was examined by H-NMR spectrum. Compare to the published spectroscopic data, and it can be established that the obtained product was 1-butyl-3-methyl-imidazolium chloride [BMIM]Cl. This compound was then used to prepare ionic liquid to extract beta-glucan from the baker's yeast (). A solution of baker's yeast and [BMIM]Cl (1:20, w/w) was stirred in 30 min at 80 C until all powder absolutely dissolved in [BMIM]Cl. Precipitates were formed in a clear solution when water was added. Pure beta-glucan as white powder was obtained with a yield of about 83.5% (compared to the beta-glucan amount contained originally in the yeast).
Cell wall -D-glucans might be divided into two subtypes following the mode of glucose linkages: long chains of ca. 1500 D-glucose units linked by (1→3)- glycosidic bonds and short chain of ca. 150 (1→6)--D-glucose units, represented of 85% and 15% of total cell wall -D-glucan, respectively 5. Titration data with KFe(CN) showed that our beta-glucan product obtained from yeast contained 95.2% -D-glucan. The 1D- and 2D- NMR spectroscopic data showed the presence of both 1,6- and 1,3--D-glucan with the proportion ratio was 1.302/7, that means 15.6% of -D-glucan was of 1,6--D-glucan, and 84.4% was 1,3--D-glucan. These facts suggested that the extraction by ionic liquid was rather selectively to obtain 1,3--D-glucan.
So far, one of the most used techniques for the extraction of β-glucan from grains and yeast is based on hot water extraction, with the inclusion of a modification of freeze-thaw cycles, with the application of high temperature, or in a combination of enzymes, acids or alkalis resulted in higher recoveries of β-glucan 4. Most of which involves two steps: (1): let the yeast autolyzed the cell wall by the enzymes available in the cell into insoluble fragments containing beta-glucan, mannoprotein, and some chitosan; (2): remove beta-glucan from impurities like proteins, starches, lipids, minerals, and other cell wall polysaccharides within the product by chemicals, enzymes and physical methods like centrifugation, ultrasound, or high pressure ...11, 12 in a series of steps are commonly used 4. However, the isolated beta-glucan from the known methods was usually mixed with impurities of the yeast cell membrane.
Nowadays, ionic liquids are attracted a lot of interest as they possess some very important properties including high polarity, high chemical, and thermal stabilities, etc., that resulted in the ionic liquids could destroy intermolecular hydrogen bonds forming the rigid triple-helix hardly-dissolved structure of yeast -D-glucan and therefore could enhance the dissolution of bio-macromolecules including cellulose, lignin, starch, chitosan and wood 5. Liu et al. reported that by using several ionic liquids like 1-ethyl-3-methylimidazolium acetate (EmimAc), 1-butyl-3-methylimidazolium acetate (BmimAc) and 1-allyl-3-methylimidazolium chloride (AmimCl) they got -D-glucan with the purity of > 81.07% 5. From the data presented in
Conclusions
In this paper, we reported that -D-glucan could be extracted from baker yeast using ionic liquid 1-butyl-3-methyl-imidazolium chloride [BMIM]Cl with the purity of 95.2%, with a yield of about 83.4% (compared to beta-glucan originally contained in the yeast)... The 1D- and 2D-NMR spectrum results confirmed that the product was the polysaccharides with β-(13) glycosidic bonds, with the β-(16)-D-glucan chain ~ 15.6%. No impurity of mannan carbohydrate was found in the product. The extraction of -D-glucan from baker's yeast with the ionic liquid [BMIM] Cl is a simple method, resulting in the production of a high purity beta-D-glucan product that is applicable in different fields as food technology, pharmaceutical, and cosmetic technology.
LIST OF ABBREVIATIONS
[BMIM]Cl - 1-butyl-3-methyl-imidazolium chloride; IUPAC- International Union of Pure and Applied Chemistry; 1D- and 2D-NMR – 1 dimensional and 2-dimensional nuclear magnetic resonance.
Authors' Contributions
Dr. Nguyen Duy Nhut was responsible for the experimental design and conduct. Assoc. Prof. Nguyen Manh Cuong revised and corrected the MS. Dr. Pham Ngoc Khanh checked references and prepared the MS. All the authors read and corrected the submitted final MS.
COMPETING INTEREST
The author(s) declare that they have no competing interests.
ACKNOWLEDGEMENTS
This work is funded by the Vietnam Academy of Science and Technology through project No.VAST04.04/17-18 and Ministry of Industry and Trade through project No.030/2019/HĐ-DA.CNHD.

