SrTiO3 nanocubes doped with ir as photocatalytic system for enhancing H2 generation from water splitting
- Faculty of Physics and Engineering Physics, VNUHCM-University of Science
- Saigon Hitech Park Labs
Abstract
Designing an effective photocatalyst for hydrogen (H2) performance under visible irradiation with a decrease the band gap energy of semiconductor has been considered as an essential aspect in boosting the performance of photocatalytic reactions. Herein, we focus on evaluating the role of doping with Ir into SrTiO3 structure fabricated by hydrothermal method for H2 generation. The crystalline characteristics the Ir-SrTiO3 photocatalyst were carried out via XRD and FE-SEM. The chemical composition and the optical properties of the Ir-SrTiO3 were classified by EDX and UV-Vis spectra, respectively. The results showcased that the dramatically improved absorbing performances of Ir/SrTiO3 specimen could be governed by the presence of Ir impurity states in the forbidden energy gap causing a decrease in energy gap of SrTiO3. This work also revealed that Ir doped into SrTiO3 exhibited excellent photocatalytic H2 evolution compared with pristine SrTiO3 (~454 and ~325 mmol.h-1.g-1 H2 production under UV and visible light irradiation, respectively). A rational photocatalytic mechanism is projected to be able provide significant awareness for further research.
INTRODUCTION
Nowadays, the rapid depletion and combustion of fossil fuels and global warming effects have become challenges increasingly. To tackle the issues of environmental crisis and usage of sustainable sources, as well as renewable technologies, have attracted the widespread concerns in recent years 1, 2, 3. As a promising hydrogen (H) energy, the hydrogen evolution reaction (HER) from water by photocatalytic water splitting has been widely used as a renewable energy source to solve the global challenges concerning the crisis of energy and the environment. In this regard, semiconductor photocatalysis applied for H evolution has been developed widely because of its outstanding contributions in environmental treatment systems 4, 5, 6. This approach bases on the formation of photogenerated electron-holes pairs from the semiconductor catalyst under suitable light irradiation. The generation of photoexcited charge carriers is changed into reactive oxygen species (ROS) to split the water to H gas formation 7, 8, 9. Among these semiconductors developed in the recent year, titanium dioxide (SrTiO) has been widely used in this field due to its non-toxic, cheap, environment-friendly, stable against corrosion and photo-corrosion and the rapid the photoinduced electron-hole pairs under light excitation, contributing enhanced photocatalytic behaviors 10, 11. Nevertheless, it's photocatalytic efficient has still hindrance related to two main drawbacks: i) SrTiO has a large forbidden energy gap (E = 3.2 eV) and is thus only promote in the UV light irradiation, which has occupied for 3–5% of total solar spectrum 12; ii) the rapid recombination rate of chare carriers has remained crucial challenges, leading to restricted the photocatalytic performance of SrTiO5, 13. To overcome these weaknesses, effective strategies to enhance photocatalytic performance has progressed at a fast pace. For example, Pt-loaded Rh-doped SrTiO exhibited a remarkably high photoelectrochemical behavior and the conversion of alcohol to efficiently form H under visible light. This result was attributed to the well-driven energy band structures of the semiconductor via Rh doping, which provided the appropriate oxidation capabilities of photogenerated holes 14. In addition, co-doping with tantalum and chromium (TiO:Ta/Cr) or nickel and tantalum or niobium (TiO:Ni/(Ta, Nb) showed a high photocatalytic performance response under visible light due to the presence of the occupied d orbitals of metals that created electron donor levels and subbands in the bandgap of TiO15, 16. Therefore, it is acknowledged that doping transition metal into semiconductor plays a significant role in the enhanced photocatalytic activity for H generation.
Among these approaches, transition metal ion doping into SrTiO nanostructures has attracted enormous attention owing to several reasons i) increasing in the visible light absorption capacity of SrTiO in the visible region due to the presence of impurities at different levels in the energy bandgap and the localized surface plasmon resonance (LSPR); ii) the fast recombination rate of photoinduced charge carriers hamper many charge transfer reactions; iii) the appearance of the Schottky barrier can be created at the interface, leading to decrease the charge recombination process. Among all kinds of transition metal co-photocatalyst, Iridium (Ir) has been considered as one of the most promising candidates on account of its ease of dispersion on some carriers and the high carrier mobility of electronic structures based on d orbital transition 17, 18. The recent studies exhibited that doping with cations into SrTiO structures suppressing backward reactions has more effective for improving the photocatalytic performance of SrTiO photocatalysts during water splitting process, attributing the presence of large active sites and the defect levels in forbidden corresponding to the interband transitions of metals and semiconductor. For example, doping with Rh, Ru, and Ir into SrTiO structures showed high activity for hydrogen evolution under the visible irradiation. Koto et al. 19 reported that the pairs of Cr-Sb, Cr-Ta, and Ni-Ta codoped SrTiO photocatalysts and evaluated the H performance. The results have shown that doping with Cr-Ta showed higher photocatalytic behavior in comparison with only Ta doping. Ma et al. showcased that H evolution rate from water based on transition metal doped SrTiO structure was higher than that of bare SrTiO20. Based on these phenomena, this work focuses on the Ir doped into SrTiO structure by a hydrothermal treatment. The results indicate that the efficiency of photocatalyst for improving the hydrogen evolution under UV-vis-light irradiation is observed by the effective separation of photogenerated charges and the appearance of matching energy levels of the midgap states in the forbidden energy gap of the semiconductor.
EXPERIMENT
Materials
Titanium tetrachloride (TiCl, Aldrich Chemical, <99%), strontium nitrate (Sr(NO), Sigma-Aldrich <99%), iridium (III) chloride hydrate (ClHIrO, Sigma-Aldrich, <99%), hydrochloric acid (HCl, Merck, <37%), and methanol (CHOH, Merck, <99.9%). The chemical reagents were used without any further purification. Double distilled water using overall the experiments were obtained from Research Laboratories of Saigon Hi-Tech Park.
Preparation of Ir-SrTiO photocatalyst
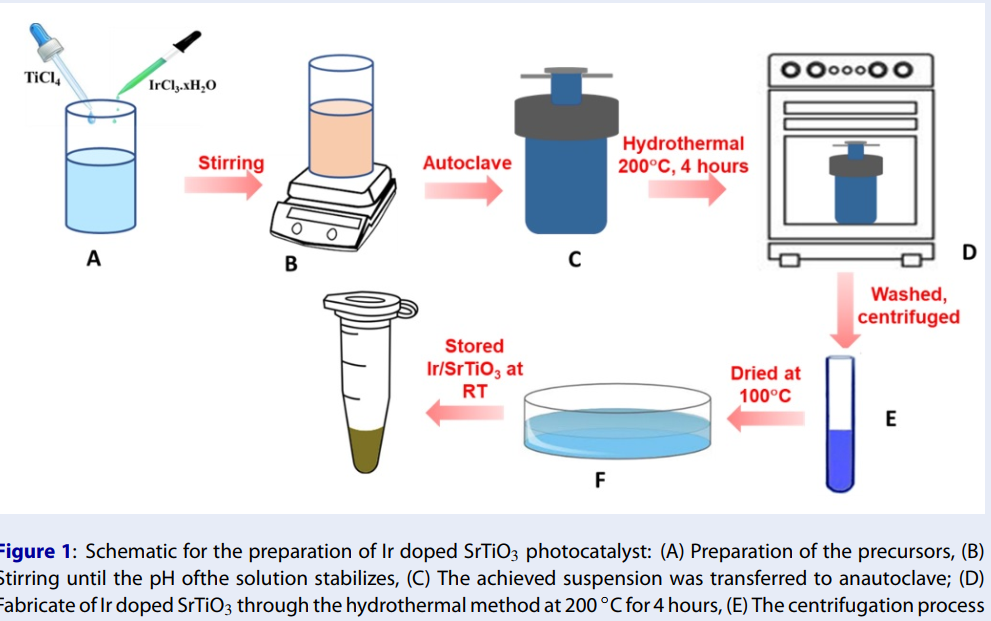
Ir-SrTiO nanoparticles have been successfully obtained using the hydrothermal method. The schematic for the preparation of Ir-SrTiO is displayed in Figure 1. First, 20 mL of a 0.5 M TiCl aqueous solution as the Ti precursor with 0.1 wt%. of IrCl.xHO used as the metal Ir-precursor was added with 60 mL of DI and stirred for 30 min, denoted a mixture. Secondly, a proper amount of Sr(NO)dissolved in 10 ml of 2M KOH solution was added to the reaction mixture. The solution was stirred for 30 minutes. The obtained suspension was then placed into an autoclave and kept at 200°C for 4 hours. The precipitates were experienced by the centrifugation process to gather the samples. After that, it was washed several times with double distilled water to eliminate the impurities. Finally, the specimen was dried under nitrogen gas flow.
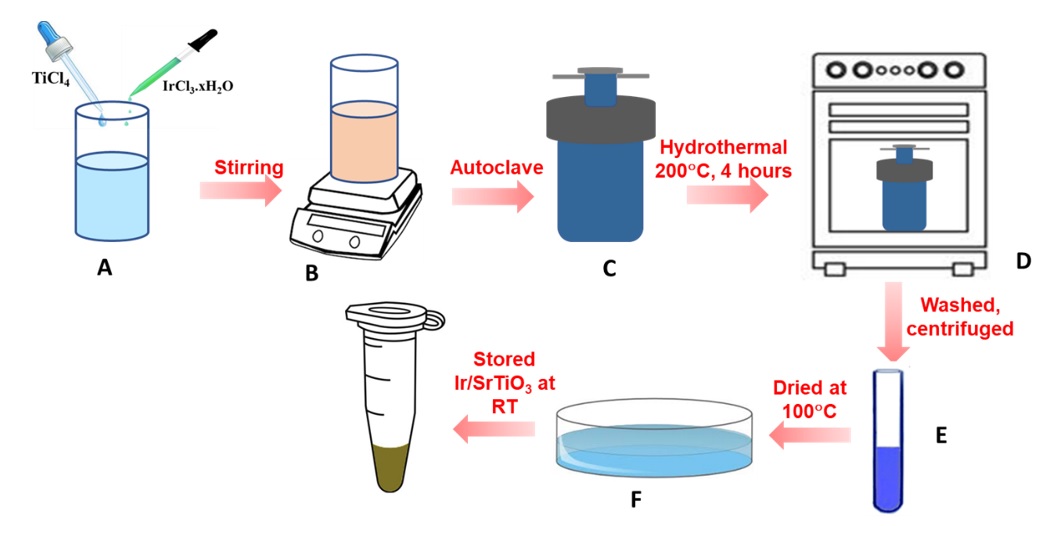
Schematic for the preparation of Ir doped SrTiO3 photocatalyst: (A) Preparation of the precursors, (B) Stirring until the pH ofthe solution stabilizes, (C) The achieved suspension was transferred to anautoclave; (D) Fabricate of Ir doped SrTiO3 through the hydrothermal method at 200 °C for 4 hours, (E) The centrifugation process to collect the sample and (F) The sample was dried under nitrogen gas flow.
Characterization
X-ray powder diffraction (XRD, a Bruker D8 ADVANCE) with a Cu Ka source was used to determine the crystalline phase of the synthesized photocatalysts. The morphology of Ir-SrTiO photocatalyst was acquired by the field emission scanning electron microscopy (FESEM, Hitachi S-4800) Its chemical components were investigated through energy-dispersive X-ray spectroscopy (EDX).
The photocatalytic behavior of H performance of water-splitting of the as-prepared Ir-SrTiO photocatalyst was performed using aqueous methanol. The samples as prepared photocatalyst were positioned at the bottom of 500 ml quartz cell with 200 ml CHOH aqueous solution. The Pyrex glass reactor was directly exposed under UV lamp (wavelength from 320 nm to 400 nm) and visible lamp as the light source. The amount of generated gaseous product was determined hourly by an off-line gas chromatography equipped with a thermal conductivity detector (TCD).
RESULTS
Characterization of the phase purity and crystallinity of the photocatalyst was thoroughly investigated by X-ray diffraction patterns. These results are displayed in Figure 2. The diffraction peaks appeared at 2q = 31°, 40°, 46°, 52.5°, 58°, 68°, and 77° which were corresponded to the diffraction of the (110), (111), (200), (210), (211), (220) and (310) crystal planes, respectively (JCPDS cards no. 74-1296) that was attributed to the cubic close-packed structure of SrTiO (marked with *). Notably, no peak corresponding to impurity states such as TiO and SrO have been observed in the spectrum. Nevertheless, Ir doped SrTiO specimen did not show the characteristic peaks of Ir due to the low quantity; similar results would be found in the previous report 21. As compared to SrTiO, Ir-SrTiO photocatalyst exhibited a slight shift towards higher 2θ scattering angles due to the replacement of Ti (0.605 Å) for Ir (0.625) 22, 23.

The crystalline characteristics through XRD patterns of as-prepared SrTiO3 and Irdoped SrTiO3 photocatalyst.
The morphology properties of pristine SrTiO and Ir-SrTiO were characterized by FESEM images as shown in Figure 3. The results reveal that a large number of aggregated cuboid nanoparticles composed of very fine primary particles was small diameter (∼50–80 nm) (Figure 2 a). Figure 2 b displays the SEM image of Ir-SrTiO obtained by the doping process of Ir. The result shows that there was no significant change observed in surface morphology compared to the pristine SrTiO after doping procedures. The results suggested that doping of Ir into SrTiO structure did not significantly affect to the morphology of photocatalyst.

The morphological features of photocatalyst (a, b) SEM image of the pristine SrTiO3, and Ir doped SrTiO3 photocatalyst, respectively.
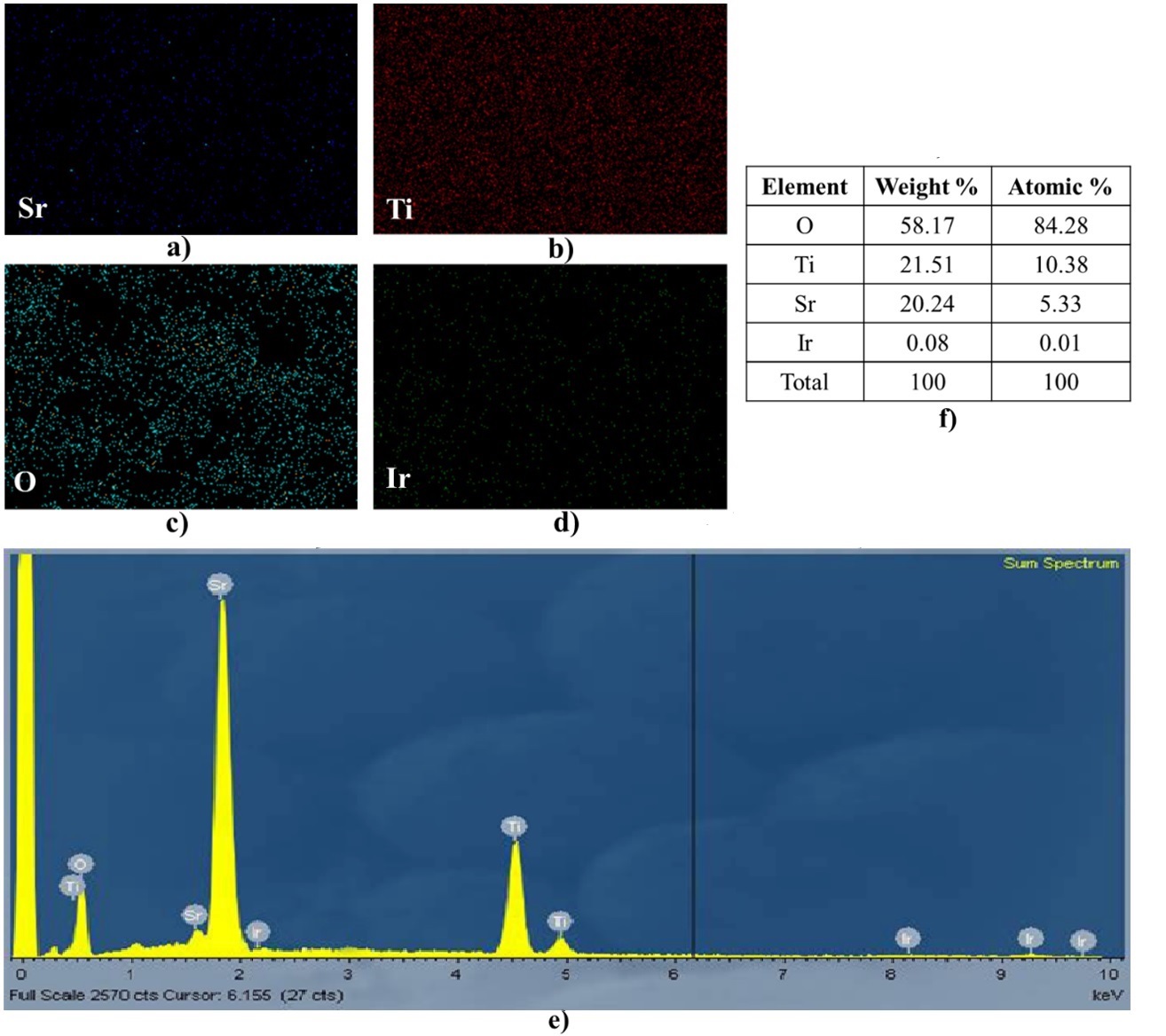
EDX elemental maps and an elemental component of the as-prepared Ir doped SrTiO3 photocatalyst. (a, b, c, d) elemental mapping of Sr, Ti, O, and Ir, respectively, (e) EDX elemental map of Ir doped SrTiO3, and (f)the composition percentage for all elements present in Ir doped SrTiO3.
Furthermore, to further verify the existence of the elemental composition of the Ir-SrTiO, EDX spectra was achieved and shown in Figure 4. The result shows that the existence of Sr, Ti, O, and Ir were detected in the photocatalyst (Figure 4 (a-d)) with the EDX elemental map and The weight percentage of elements analyzed by EDX corresponding wt.% as shown in the table Figure 4 (e,f). The appearance of Ir species indicated that Ir species were successfully deposited onto SrTiO structures. The peak intensity corresponding to the concentration level of the element in the SrTiO was observed. Despite of low doping concentration of Ir, the peaks showed to be homogenously anchored in the photocatalyst structure.

The absorption characteristics of the photocatalyst. (a) The UV–Vis absorption spectra and (b) plot of (ahn)1/2 vs. photon energy of pristine SrTiO3 andas-prepared Ir doped SrTiO3.
The UV–vis absorbance spectra of pristine SrTiO and Ir-SrTiO photocatalysts were studied to reveal the optical properties of a sample. The characterization of absorption and the energy bandgap was determined by Kubelka-Munk equation, as depicted in Figure 5. It can be observed that the SrTiO structures unveil a strong absorption edge (Figure 5 a) related to the large bandgap energy of 3.2 eV (Figure 5 b), which was assigned to the transfer of valence band to the conduction band. These results were consistent with previous works 24. The absorption band of Ir doped into SrTiO sample shows a significant shift to longer wavelengths at nearly 478 nm with respect to the bandgap of 2.1 eV (Figure 5 b), which can be assigned to some reasons: i) electron transition from the Ir occupied levels present above the valance band to the conduction band of SrTiO causing the expansion of the absorption edge in the visible-light-driven; ii) the transition from Ir 3d electrons to the conduction band of SrTiO. As a result, a narrower bandgap energy was obtained after doping with Ir into SrTiO. The results are similar to previously published data 25, 26.

Photocatalytic hydrogen generation of the fabricated photocatalyst under the different reaction conditions (a) under UV, (b) visible light irradiation, and (c) recycling stability experiments for photocatalytic H2 performance under visible region after 4 cycles of SrTiO3 and Ir doped SrTiO3 photocatalyst.
To assess the role of doping with Ir on photocatalytic H evolution with SrTiO. The performance of photocatalytic H production in the SrTiO and Ir-SrTiO was investigated using a CHOH aqueous solution as a hole scavenger and direct exposure to UV and visible light, as presented in Figure 6. It can be clearly seen that the photocatalytic performances for hydrogen generation of Ir-SrTiO exhibited a superior compared with that of pristine SrTiO under both UV light and visible light. Especially, it can be found that Ir-SrTiO photocatalyst possesses a dramatic H evolution with the amount of ~325 mmol.h.g, which was more than 14-fold as much as that of SrTiO under visible light, whereas, no H production is detected under visible illumination. This could be assigned to the fact that the pristine SrTiO photocatalyst is not activated under visible regime irradiation because of the large bandgap energy that is only driven by ultraviolet light, leading to low H efficacy. On the other hand, doping Ir into SrTiO structure, the photocatalyst would be activated under the visible light, thanks to the reduced forbidden energy gap related to the existence of impurity levels in the bandgap energy. As a result, the photoinduced charge carriers made the redox reactions to produce H gas; thus, the photocatalytic H generation would be significantly improved under the visible region illumination. The recycling stability of the photocatalyst is also an essential factor in evaluating its performance. Hence, the reusability of photocatalyst for H evolution was performed through repeated cycles under visible light irradiation. As displayed in Figure 6, the H production activities of Ir-SrTiO stayed constant after four cycles, indicating the high stability of photocatalyst toward potential practices in renewable energy technologies.
DISCUSSION
Based on a series of characterization, an appropriate reaction mechanism for photocatalytic H generation regarding the generation of photoinduced charge carriers have been proposed. When the photocatalyst is activated by photon energy equal or greater than their forbidden energy gap, photogenerated electron–hole pairs are excited. These electrons undergo photocatalytic decomposition of water reduction and reduce H to produce H molecules. On the other hand, the photoinduced holes at the valance band react quickly with HO to form hydroxyl radicals in a consecutive reaction route. Compared with the photocatalytic activity for H generation of bare SrTiO, the H production of Ir doped SrTiO specimen enhanced significantly, which means that the doping with Ir could decrease the forbidden band of the semiconductor as shown in Figure 5 and prevent the rapid recombination process of photoinduced charge carriers, while most photogenerated charge carriers in pristine SrTiO could be quickly recombined, resulting in low photocatalytic behavior. Moreover, doping with Ir into SrTiO structure could appear some defects in the forbidden band as shown in the outcomes of UV-Vis section; these defects are believed to be the active sites in photocatalytic reaction contributing the enhancement of H photocatalytic. In the whole process, a positive effect of charge carrier performance related to the defects providing effective separation of photogenerated chare carriers are obtained. There are no detailed studies for the water-splitting efficiency in Ir-SrTiO samples. In recent research, a similar kind of trend was observed by Fadlallah et al. for cation codoped SrTiO photocatalysts for water splitting. Photocatalytic activity of SrTiO codoped metal transition such as Rh, V, Sb significantly improved as compared to pristine SrTiO due to the reduction of the bandgap energy of host structure 27. Ma et al. reported that the H production from water splitting of transition metal-doped SrTiO photocatalysts exhibited higher than that of pristine SrTiO due to the presence of sub-bandgap states in the SrTiO lattice under visible light 28. Therefore, the results of our research showcase the high H evolution at a wavelength of 590 nm (Ir doped SrTiO specimen exhibited both UV light and visible light irradiation). These results from this research provide an insight into the role of the photocatalyst in potential practices for renewable energy technologies.
CONCLUSION
In conclusion, Ir elements have successfully doped into the perovskite structure of SrTiO by hydrothermal method. The phase content and structure characterization did not change dramatically after doing with Ir. Compared to those of pristine SrTiO, the improved absorption capability of Ir-SrTiO specimen in the visible light was observed. Moreover, doping Ir into SrTiO structure exhibited a dramatically improved visible-light-driven photocatalytic H evolution performance. The H evolution could reach up to ~325 mmol.h.g under visible light irradiation after 180 minutes. This may be assigned to the productive charge separation of photogenerated electron-hole pairs and the reduction of the forbidden energy gap of SrTiO. In general, these findings supply an insight into constructing a photocatalytic system by doping Ir into the semiconductor to coordinate the charge separation efficiency for clean energy production.
COMPETING INTERESTS
The authors declare that there is no conflict of interest regarding the publication of this article.
AUTHORS’ CONTRIBUTIONS
Ton Nu Quynh Trang has conceived of the present idea, carried out and written the manuscript with support from Vu Thi Hanh Thu
Tieu Tu Doanh and Do Thanh Sinh has supported the analytical techniques
ACKNOWLEDGMENTS
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.99-2018.364.

