Preparation of Heterogeneous Fenton-Type Nano Catalysts and Their Application to Methylene Blue Degradation
- University of Science, Ho Chi Minh City, Vietnam
Abstract
Introduction: Iron-based nanocatalysts are known as a new generation heterogeneous Fenton catalyst, replacing the traditional Fenton catalyst system which has many disadvantages in experimental processes and industrial applications. In this study, we focused on the preparation of iron nanoparticles and their use when embedded in traditional supports, as well as tested their catalytic activity by modified Fenton-type oxidation of methylene blue (MB) substrate.
Method: Scanning Electron Microscope (SEM), Transmission Electron Microscopy (TEM), X-ray diffraction (XRD) and UV-vis were used for physio-chemical characterization of the catalysts.
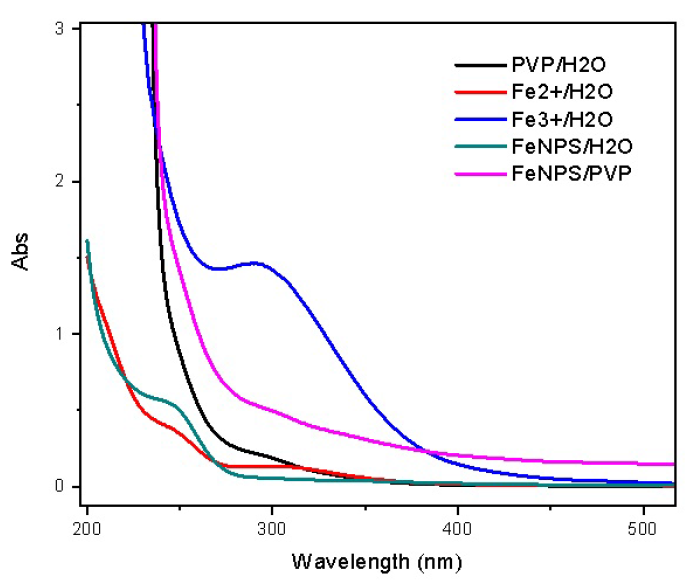
Results: Iron nanoparticles were obtained in the reduction of iron salt by sodium borohydride (NaBH4), with particle size in the range of 4-5 nm. Fe-X (X represents C, Bentonite, Al2O3, or ZnO) was synthesized in high yield and applied to the Fenton oxidation of MB; approximately 99% conversion was observed in the case of Fe-C.
Conclusion: Supported iron nanoparticles are active catalysts for the oxidation of MB; however, there are limitations if pH is above 3.

