The efficiency of short sintering time on thermoelectric properties of delafossite CuCr0:85Mg0:15O2 ceramics
- Laboratory of Advanced Materials, University of Science, Ho Chi Minh City, Viet Nam
- Vietnam National University Ho Chi Minh City, Viet Nam
- Center for Innovative Materials and Architectures, Ho Chi Minh City, Viet Nam
- 4Department of Mathematics and Physics, University of Information Technology, Ho Chi Minh City, Viet Nam
- 2Vietnam National University Ho Chi Minh City, Viet Nam
Abstract
Introduction: Harvesting the waste heat emitted from the activities of humanity based on thermoelectric devices is an appropriate way to reduce the overconsumption of fossil fuel nowadays.
Methods: In this work, CuCr0:85Mg0:15O2 compounds prepared by conventional solid-state reaction method were investigated to find out that the short sintering time is enough for thermoelectric applications, directly low the cost of the devices.
Results and Conclusion: We find out that there is a significant change in the crystal structure, the chemical state, and thermoelectric properties along with the increase of the sintering time, but eventually, the dimensionless figure of merit ZT is almost constant regardless of the long or short sintering time which means that the increase of electrical conductivity will compromise the increase of thermal conductivity. The highest ZT value is 0.03 measured at 500 oC for both samples prepared at the sintering time of 3 and 12 hours.
INTRODUCTION
Thermoelectric materials have recently emerged as a potential candidate for harvesting the waste heat from artificial sources: vehicles using the robust engines, thermal power plants, … or natural sources: geothermal or solar energy. Thermoelectric devices convert heat energy based on the dimensionless figure of merit ZT = σ.S.T/(κ + κ) 1, where σ (S/cm) is electrical conductivity, S (µV/K) is Seebeck coefficient, κ is electron thermal conductivity and κ is lattice thermal conductivity. Therefore, a material that serves for thermoelectric device needs the ZT value is as high as possible. Hence, the transport parameters (Seebeck coefficient, electrical, and thermal conductivity) need to be improved. However, these transport parameters commonly vanish to each other (e.g., the increase of electrical conductivity as elevating temperature gives rise to the decrease of Seebeck coefficient and the growth of thermal conductivity because of bipolar effect 1). Therefore, it is important to explore a material that could compromise those transport parameters.
Recently, oxide materials emerge as a potential candidate for thermoelectric applications due to their advantages: (i) the stability of oxide compounds when it is exposed on ambient air at high temperature led to enhance the ZT value as following equation 1; (ii) the raw materials have low cost and environmental friendliness 2. There are a number of thermoelectric oxide materials reported with high thermoelectric performance, such as SrTiO, CaCoO, NaCoO, ZnO, InO, and BiCuSeO 3. Among them, the layered cobalt oxides (CaCoO and NaCoO) are known as good -type thermoelectric oxide material at high temperatures around 700 – 1000 K 2, 4. However, it is noted that NaCoO will be decomposed into insulating Co(OH) as being exposed in a high humidity environment 2. In the case of the CaCoO compound, the anisotropic electric properties are caused by its crystal structure and the less densification because of the large difference of temperature between the eutectic point and the stable range of CaCoO phase are the two main disadvantages of this material5. Delafossite, known as an inherited p-type material, has the layered-type structure belonged to cobaltite oxide family like CaCoO and NaCoO. The crystal structure of delafossite which has the general chemical formular is ABO (where A is Cu, Ag, Pd or Pt and B is group III elements in the periodic table) is the alternation of A-plane and BO edge-shared octahedral layers. Therefore, it is expected that this material has the thermoelectric performance similar to CaCoO or NaCoO
Many efforts have been made to enhance the thermoelectric properties of delafossite materials in which doping is a popular method. In the family of delafossite materials, Mg-doped CuCrO have been known as the highest conductivity with a value of 278 S/cm 6 so far. Besides, Mg-doped CuCrO also has a high Seebeck coefficient with the value in the range from 200 – 450 µV/K 7, 8, 9, 10. However, this material encounters with the difficulty that the Mg doping could significantly improve electrical conductivity, whereas this gives rise to the dramatical decrease of the Seebeck coefficient 10, 11 and causes the increase of the thermal conductivity 11. For example, Okuda et al.10 prepared CuCrMgO compounds with various Mg concentrations (0 ≤ x ≤ 0.04) and found out a dramatic decrease of Seebeck coefficient from 350 to 70 µV/K with a small increase of Mg concentration from x = 0 to 0.03, respectively. In another report, Hayashi et al. 11 found out an increase of thermal conductivity from ~ 6 to ~ 7 W/m.K of CuCrMgNiO compounds as x increase from 0 to 0.05, respectively. In the previous report12, we systematically investigated the CuCrMgO (0 ≤ x ≤ 0.3) compounds. We found out that the high Mg doping concentration (x = 0.15) could significantly increase the electrical conductivity and decrease the thermal conductivity due to the appearance of the multi-scale defects (copper vacancies, oxygen interstitial, secondary phases like CuO, MgCrO) in the compound.
The solid-state reaction method is commonly used to synthesis the bulk Mg-doped CuCrO materials due to its simplification and the ability to build large-scale production. The reports related to Mg-doped CuCrO compounds used for thermoelectric applications using the conventional solid-state reaction method almost sintered the pellets for a long time of sintering (conventionally over 10 hours)7, 8, 10, 11, 13, 14, 15, 16, 17. In the industry, reducing the time to make a product is very important to low the cost. In this work, we investigate the effects of the sintering time on thermoelectric properties of CuCrMgO compounds to consider the long sintering time as previously reported literature whether it is important or not for this compound.
METHODS
The CuCrMgO bulk samples were prepared by using the conventional solid-state reaction method. CuO, CrO and MgO powders (the purity > 99% for all) were used as precursor materials. These materials are mixed by distilled water and ground by a planetary ball mill for grinding the mixed powder in alumina oxide (AlO) mortar for 5 hours. The mixture obtained after the grinding process was put into the oven with a temperature of 120 C for 24 hours to evaporate the water. After drying, the powder will be ground by hand with an agate mortar and then pressed to form a rectangular shape with a size of 30x30x6 mm. This green compact will be sintered at 1200C with sintering times of 3 hours and 12 hours.
The compounds' crystal structure was investigated using powder x-ray diffraction (XRD) method on the Bruker D8 Advanced system. A small specimen was extracted from the rectangular pellets and was ground by hand on an agate mortar. A 200-mesh sieve filtered the powder to obtain the particle whose diameter is smaller than 74 microns. The interval step of XRD is 0.02 and the period for a data point is 0.25 seconds. The morphology of a crystal grain was imaged by Field Emission Scanning Electron Microscopy (FESEM) (Hitachi S-4800) from a surface of a specimen broken from the pellets. The phases of bulk samples were detected by using a JEM2100F high-resolution transmission electron microscope (HRTEM). X-ray photoelectron spectroscopy (XPS) was conducted to investigate the chemical state of the compound by using the K-alpha XPS system (Thermo Scientific) with monochromatic Al Kα-1486.6 eV. The room-temperature Hall effect based on the Van der Pauw method was used to determine the carrier concentration, hole mobility, and conductivity of the sheet with the dimension of 10×10×0.5 mm which was cut from the initial rectangular pellets. The Seebeck coefficient and electrical conductivity were obtained from the LSR-3 system (Linseis GmbH, Germany) in the temperature range of 50 – 500 C. An LFA-457 MicroFlash Thermal Analyzer (NETZSCH, Germany) was used to determine the total thermal conductivity.
RESULTS AND DISCUSSIONS
Figure 1 depicts the XRD diagrams of CuCrMgO compounds prepared at the sintering temperature of 1200 C with the sintering times of 3 and 12 hours. Generally, there is seemingly no significant difference between two XRD patterns which mainly appear in the peaks of the delafossite phase (PDF # 74-0983). Besides, MgCrO and CuO phase were also revealed based on the XRD standards: PDF #77 – 0007 and #45 – 0937, respectively. There is no trace of the raw materials, including CuO, CrO and MgO in the XRD patterns, indicating that the compounds were completely converted into CuCrO, MgCrO and CuO phase at the sintering temperature of 1200 C. In the literatures, CuCrO material is proved that this compound is successfully formed at the sintering temperature more fabulous than 1000 C from the raw materials of CuO and CrO7, 15, 17, 18. The formation of MgCrO phase in the compounds relates to the solubility of Mg concentration in CuCrO material, which has a low limited solubility under 1 % 8, 19. In this work, the un-stoichiometry of Cu : Cr ratio (1 : 0.85) of the samples compared with an ideal delafossite material gives rise to the the CuO phase.
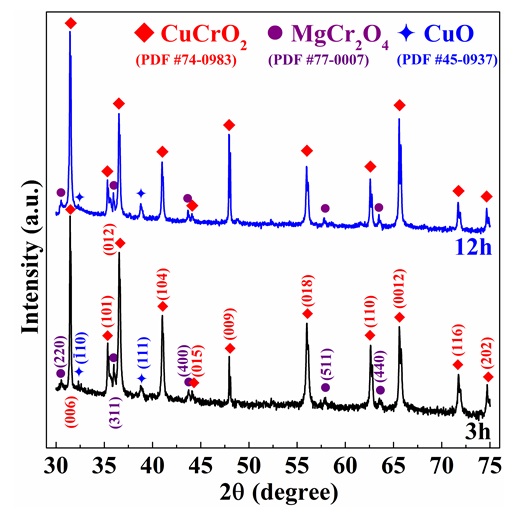
XRD patterns of CuCr0.85Mg0.15O2 samples prepared at two different sintering time. The CuCrO2, CuO and MgCr2O4 phases are symbolled by the red rhombus, blue four-sided star and purple circle based on the PDF files: # 74-0983, # 45-0937 and # 77-0007, respectively. XRD pattern of 3h sample was reprinted from the Ref.
Structural parameters extracted from XRD results. The
|
Structural parameters |
CuCr0.85Mg0.15O2 @ 3h |
CuCr0.85Mg0.15O2 @ 12h |
|
|
2.965 |
2.966 |
|
|
17.055 |
17.054 |
|
(006) crystallite size (nm) [CuCrO2] |
166 |
205 |
|
(311) crystallite size (nm) [MgCr2O4] |
109 |
92 |
|
(111) crystallite size (nm) [CuO] |
31 |
42 |
|
Mass density (g/cm3) |
3.71 |
3.91 |
To shed more light on the effects of sintering temperature on structural properties of CuCrMgO samples, the and lattice parameters of CuCrO phase, the crystallite size (, where is the Miller indices) of (006), (311), and (111) planes which represent for CuCrO, MgCrO and CuO, respectively, are obtained from XRD data and listed in
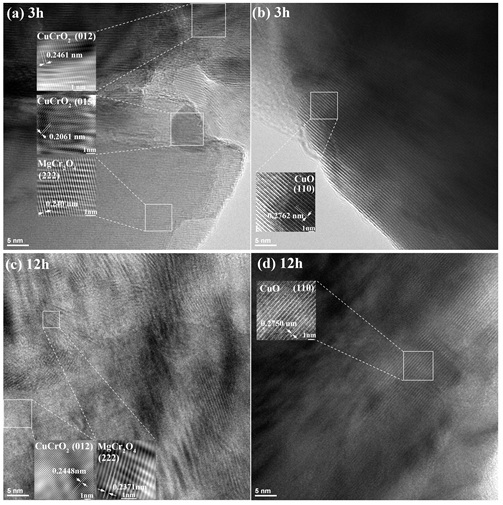
HRTEM images of CuCr0.85Mg0.15O2 compounds sintered at sintering time of (a, b) 3 hours and (c, d) 12 hours.
HRTEM micrographs of CuCrMgO compounds, as seen in Figure 2 was used as a supplemental tool for the X-ray diffraction method to detect the existence of the multi-phases in the compounds. The co-existence of CuCrO, MgCrO, and CuO phases in bulk samples is clearly observed regardless of the long or short sintering time. In addition, interplanar spacing between crystal planes of those phases has a reducing trend which implies that the compound become more denser as increasing the sintering time. This result shows the consistent increase of mass density and the sintering time as seen in

FESEM images of CuCr0.85Mg0.15O2 compounds prepared at 1200 oC with the sintering time of (a) 3 hours and (b) 12 hours. FESEM image of 3h sample was reprinted from the Ref.
The surface morphology of the CuCrMgO compounds sintered for different dwell times can be observed in Figure 3. In both images, the CuCrO phase can clearly be observed via the grains with the face like “terraces” because the delafossite material has the layer structure 23, 24, 25. Besides, for the sample prepared with low dwell time, grains with the shape of the octahedron (typical shape of spinel MgCrO) can be distinctly observed and evenly distributed in the compound, while the octahedral grains are difficult to find in the image of the sample with high dwell time. It is difficult to observe the existence of the CuO phase by FESEM images due to small contributions, as seen in XRD results. Therefore, from FESEM images, it can be clearly seen the predominance of delafossite phase in the compounds which is the consistence of XRD results.
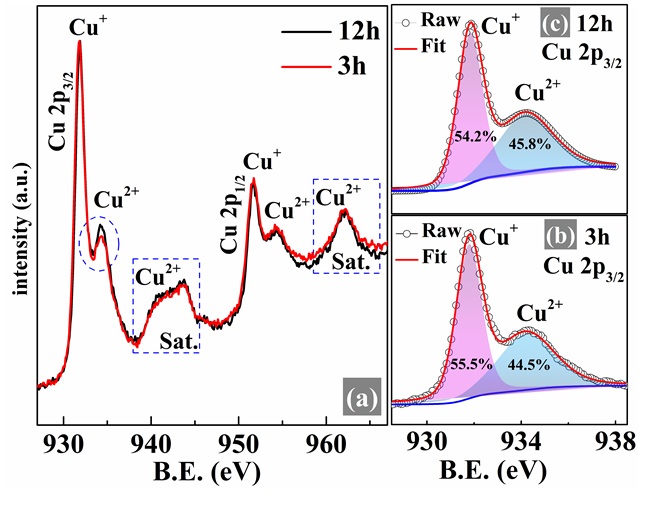
XPS curves of (a) Cu 2p of CuCr0.85Mg0.15O2 compounds prepared at various sintering times. Deconvoluted XPS spectra of Cu 2p3/2 of (b) 3h and (c) 12h samples. The dash blue circle and rectangular depict the region of the Cu2+ state in the Cu 2p3/2 peak and the satellite (sat.) peaks. XPS results of 3h sample were reprinted from Ref.
The Cu 2p photoelectron spectra of the CuCrMgO compounds and its Cu 2p deconvoluted spectra are shown in Figure 4. The XPS spectra of Cu 2p in Figure 4(a) show an insignificant difference between the compounds and witness the simultaneous existence of Cu and Cu ion states. Besides, the appearance of a broadband located at ca. 940 – 945 eV and ca. 960 – 965 eV named “satellite” peaks indicates the contribution of the Cu ion state26. The blue dash circle in Figure 4(a) indicates that the Cu state tends to increase with sintering time. To get more information on the change of the Cu state, the Cu 2p was deconvoluted into two peaks of Cu and Cu as seen in Figure 4 (b) and (c), respectively. The area percentage of Cu state has a reducing trend with the increase of sintering time, while Cu has the opposite trend. The mixed-valence states Cu/Cu relates to the electrical transport mechanism in Cu-based materials27. As listed in
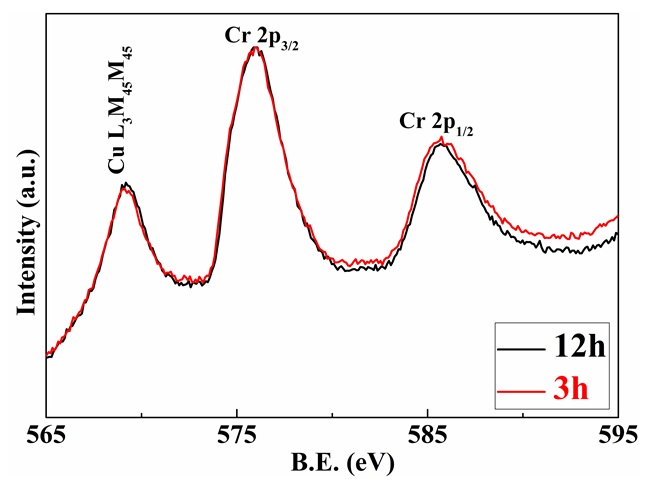
XPS spectra of Cr 2p of CuCr0.85Mg0.15O2 compounds prepared at various sintering times. XPS results of 3h sample were reprinted from Ref.
Figure 5 depicts the dependence of Cr 2p ion state of CuCrMgO compounds on the sintering time. There is no difference between the line of two samples, which indicates that the sintering time does not give rise to the change in the Cr ion state. Besides, the appearance of Cu LMM at the binding energy of 569.2 eV indicates that the delafossite phase is contaminated by copper oxide28.
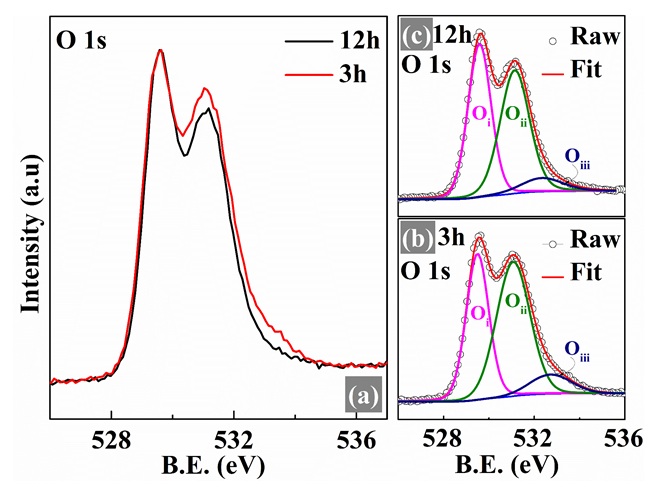
XPS spectra of (a) O 1s of CuCr0.85Mg0.15O2 compounds prepared at various sintering times. Deconvoluted XPS spectra of O 1s of (b) 3h and (b) 12h samples. XPS results of 3h sample were reprinted from Ref.
In comparison with XPS spectra of Cu 2p and Cr 2p, the XPS spectra of O 1s shown in Figure 6(a) has a significant difference between two sintering time. To get more detailed information, the XPS spectra of O 1s were deconvoluted into three peaks: (O) is assigned to the oxygen in its position of crystal structure which bonds with metal atoms, (O) relates to the intercalation of oxygen between the Cu-plane and CrO plane in delafossite structure, and (O) is surface absorbed oxygen22, 29, 30. The deconvoluted results are shown in Figure 6(b) and (c), and their details are listed in
Binding energy (B.E.), fullwidth at half maximum (FWHM), and area percentage (%) of Cu 2p3/2, and O 1s peaks extracted from XPS spectra by deconvoluting based on Gaussian – Lorentzian mixed function. The area ratio of [Cu+/2+] was calculated by using the area of each peak.
|
3h |
12h | |||||
|
B.E. (eV) |
FWHM (eV) |
% |
B.E. (eV) |
FWHM (eV) |
% | |
|
Cu 2p3/2 |
931.8 [Cu+] |
1.3 |
55.5 |
931.8 |
1.3 |
54.2 |
|
934.2 [Cu2+] |
2.9 |
44.5 |
934.2 |
2.7 |
45.8 | |
|
[Cu+/2+] |
1.25 |
1.19 | ||||
|
O 1s |
529.5 (i) |
1.1 |
36.5 |
529.5 |
1.1 |
41.4 |
|
531.1 (ii) |
1.8 |
55.2 |
531.1 |
1.5 |
53.7 | |
|
532.5 (iii) |
2.1 |
8.3 |
532.5 |
2.1 |
4.9 | |
The room temperature hole concentration, hole mobility, and conductivity of CuCr0.85Mg0.15O2 compounds prepared at various sintering times. Hall measurement results of 3h sample were reprinted from Ref.
|
Sample |
Hole concentration (×1019 cm-3) |
Hole mobility (cm2/Vs) |
Conductivity (S/cm) |
|
CuCr0.85Mg0.15O2 @ 3h |
3.9 |
0.49 |
3.1 |
|
CuCr0.85Mg0.15O2 @ 12h |
5.3 |
0.55 |
4.7 |
The hole concentration, hole mobility, and conductivity of CuCrMgO compounds prepared at the sintering time of 3 and 12 hours are listed in

Thermoelectric properties of of CuCr0.85Mg0.15O2 compounds. (a) The electrical conductivity, (b) Seebeck coefficient, and (c) power factor of samples prepared at sintering time of 3h and 12h as a function of measuring temperature. The electrical conductivity, Seebeck coefficient, and power factor results of 3h sample was reprinted from Ref.
The electrical conductivity (σ), Seebeck coefficient (S), and power factor (PF) of CuCrMgO compounds depending on measuring temperature in Figure 7 show a general picture of the effects of sintering time on the electrical properties. The EC in Figure 7(a) has a slight increase in sintering time and measuring temperature. The increase of σ with measuring temperature indicates that the σ of the compounds behaves as semiconductors. The elevation of sintering time enhances the σ because the oxygen vacancies could be passivated by oxygen (O). The S in Figure 7(b) is positive values which indicate that the compounds are p-type semiconductors. The S values of the compounds have a similar value as the measuring temperature is lower than 300 C. However, at a high measuring temperature (> 300 C), the S values of 12h sample are higher than that of 3h sample. The power factor directly depends on σ and S (i.e., PF = σ.S), and hence, in Figure 7(c), the PF values of 12h sample are higher than that of 3h sample because of electrical conductivity.
Figure 8 shows the correlation between the electron (κ), lattice (κ), and total thermal conductivity (κ) and measuring the temperature of CuCrMgO compounds prepared at sintering time of 3h and 12h. Figure 8(a) shows that the electron thermal conductivity exhibits a small increase with the sintering time, but its contribution to the total thermal conductivity is insignificant. The line-shape of κ and κ match well together, which indicates that the κ is mainly governed by the κ. The κ increases approximately 10 % with the rise of sintering time from 3 hours to 12 hours. The increase of κ relates to the change of crystallite size of CuCrO and MgCrO phases. More specifically, CuCrO phase has high thermal conductivity (5 – 10 W/mK)11, 24, while MgCrO phase has relatively low thermal conductivity (~ 2 W/mK) 12, 31. Therefore, the increase and decrease of crystallite size of CuCrO and MgCrO phase, respectively, give rise to the increase of the κ.

Temperature dependence of (a) the electrical, (b) the lattice, and (c) total thermal conductivity of CuCr0.85Mg0.15O2 compounds prepared at sintering time of 3h and 12h.

Temperature dependence of the dimensionless figure of merit (ZT) of CuCr0.85Mg0.15O2 compounds prepared at different sintering times.
There is a slight increase of PF, as seen in Figure 7 along with the rise of sintering time. Still, the thermal conductivity shown in Figure 8 also increases, which gives rise to the almost unchanged ZT values despite a fourfold increase of the sintering time as depicted in Figure 9. The thermal conductivity is almost constant with the measuring temperature, and therefore the rise of ZT is dependent on electrical conductivity. The compounds reach the highest ZT value of 3 × 10 at the measuring temperature of 500 C regardless of the short or long period of the sintering time. This value of ZT is relatively high compared to published reports related to doped CuCrO32 or other cuprous delafossite materials22 at a similar measuring temperature.
CONCLUSIONS
The effects of the sintering time on the crystal structure, the chemical state, and the thermoelectric properties of CuCrMgO compounds prepared at a sintering temperature of 1200 C have been investigated in this work. The XRD results show an increase of crystallite size of CuCrO and CuO phase. In contrast, the crystallite size of the MgCrO phase shows the reducing tendency, which gives rise to the increase of total thermal conductivity. However, the ZT value is almost constant due to the rise of electrical conductivity. Generally, based on ZT results, it is observed that in the case of CuCrMgO compounds, the short sintering time (3 hours) is enough for synthesizing materials used for thermoelectric applications, which in turn increase the economic performance.
LIST OF ABBREVIATIONS
ZT: dimensionless figure of merit
XRD: powder x-ray diffraction
FESEM: Field Emission Scanning Electron Microscopy
HRTEM: high-resolution transmission electron microscope
XPS: X-ray photoelectron spectroscopy
B.E.: Binding energy
FWHM: fullwidth at half maximum
PDF: Powder diffraction file
Authors Contribution
Dung Van Hoang: Data curation, Methodology; Investigation, Writing-original draft; Truong Huu Nguyen: Data curation, Formal Analysis; Anh Tuan Thanh Pham: Data curation, Formal Analysis; Thu Bao Nguyen Le: Data curation, Formal Analysis; Vinh Cao Tran: Data curation, Supervision; Thang Bach Phan: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Vietnam Ministry of Science and Technology under grant number ÐTÐL.CN-23/18.

