Market research and fabrication of low-cost disinfectant spray for pets using silver nanoparticles
- School of Biomedical Engineering – International University Vietnam National Universities HCMC, Viet Nam
- School of Biomedical Engineering, International University, Vietnam National University Ho Chi Minh city
Abstract
Introduction: Silver nanoparticles (AgNPs) have been widely studied and applied to many fields. They are known for their superior antibacterial properties. In this study, AgNPs are particularly studied and used in veterinary medicines, typically for pets such as dogs, cats and rabbits. Unlike humans, these pets often have skin problems since their skin is an ideal environment for bacteria and viruses to grow. Normally, using existing cleaning products for pets such as shampoo or special skin treatment products would be a good option to keep the pets safe and clean. Nevertheless, such products are inconvenient to use daily, or those products are quite expensive since they are mostly imported from foreign countries.
Methods: To cope with this issue, a low-cost disinfectant spray for pets using silver nanoparticles has been developed. The very first step of this study is to understand market demand and customer insight into this product. Based on market research, it would be visible whether the fabrication of the disinfectant spray is necessary. Furthermore, the study focuses on the chemical synthesis approach where the reaction efficiency is maximized while the use of chemicals is minimized. Additionally, the produced product was characterized and tested in vitro with Staphylococcus aureus and Escherichia coli.
Results: The result shows that this disinfectant product is topically and orally safe for pets, and it can eliminate over 90% of the bacteria within one minute.
Conclusion: Overall, a disinfectant spray for pets using chemically synthesized silver nanoparticles has been successfully developed, and this spray presents a potential domestic product for pets.
INTRODUCTION
Silver nanoparticles range in size from 1-100 nanometers1. They have a very large surface area, which helps to increase their exposure to bacteria. AgNPs kill bacteria by many mechanisms, affecting many processes, including the structure of the cell membrane, the ability to transport the membrane, metabolic processes and the impact on the genetic material of the microorganism2, 3, 4. Therefore, bacteria are almost incapable of resisting silver nanoparticles.
More specifically, the adhesion of silver nanoparticles to bacterial cell membranes changes the membrane properties and the transport of substances across the bacterial cell membranes1, 3. This adhesion depends on the concentration, shape, and size of the nanoparticles. The small size and large surface area of AgNPs increase their adhesion to the surface of cells.
Additionally, silver's antibacterial properties are derived from the chemical properties of silver ions. This ion has the ability to bind strongly with peptidoglycan, the constituent of the cell wall of bacteria, and inhibit the ability to transport oxygen into the cell, leading to the paralysis of the bacteria2. Animal cells of the higher group of organisms have completely different protective membranes compared to single-cell microbial cells. Advanced cells have two layers of durable double-bonded lipoprotein that are electronically capable and thus do not allow Ag silver ions to penetrate, so the cell is not damaged when exposed to silver ions. This means that silver nanoparticles are completely harmless to humans and animals in general due to their more stable and thicker membrane structure than pathogenic microorganisms such as fungi and bacteria. It can be said that the effect of silver ions here is not pathologically specific like an antibiotic but is structurally specific. Therefore, any cell that does not have a chemically stable protective film is easily affected by silver3, 4. At the same time, silver acts as a catalyst, so it is less wasted during use.
Furthermore, after entering the body, Ag turns into Ag acting on the protective membrane of pathogenic bacterial cells it will go inside the cell and react with the SH group of the enzyme molecule to metabolize oxygen and inactivate this enzyme, which leads to inhibition of the respiration of bacterial cells2, 3, 4. Silver ions also inhibit the nitrogen, phosphorus, and sulfur cycles of nitrate bacteria. In addition, it inactivates other metabolic enzymes containing thiol, sulfur, etc.
Several other antibacterial mechanisms are based on adsorption theory: inactivation of mycobacteria as a result of the electrostatic interaction between the negatively charged surface of the cell and the adsorbed silver ions onto the bacterial surface, which then penetrates inside the cell and neutralizes them1.
Finally, nanosilver also promotes wound healing through fibroblast stimulation, increasing collagen synthesis. Therefore, the skin of pets can recover faster and return to its original state of health5.
As AgNPs are technically very small particles of silver, there are several ways to synthesize these particles. There are currently three principal approaches: physical methods, chemical methods, and biological methods6, 7. These three methods can be utilized independently or together to yield silver nanoparticles.
The most essential physical methods for synthesizing AgNPs include evaporation-condensation, laser ablation, electrical irradiation, gamma irradiation, and lithography. The mechanism of this approach is to breakdown silver as a whole into very small pieces of silver nanoparticles using physical factors such as heat, radiation, and rays6, 7.
The most commonly used technique to synthesize AgNPs is the chemical method, which is based on the principle of chemical reduction by organic or inorganic reducing agents6, 7. Some different reducing agents, such as sodium citrate, ascorbate, sodium borohydride (NaBH), elemental hydrogen, polyol process, Tollens reagent, N,N-dimethylformamide (DMF), and poly(ethylene glycol)-block copolymers, are used for the reduction of Ag+ silver ions in aqueous or nonaqueous solutions. These reducing agents reduce Ag and lead to the formation of metallic silver Ag, which is followed by agglomeration into oligomeric clusters. These clusters eventually form metallic colloidal silver particles. Moreover, it is crucial to use stabilizing agents to prevent the agglomeration of the particles by using polymeric compounds such as poly (vinyl alcohol), poly (vinylpyrrolidone), poly (ethylene glycol), poly (methacrylic acid), and polymethylmethacrylate6. Furthermore, this approach can be used together with physical methods (physicochemical methods) to support the process of making silver nanoparticles.
Finally, the biological synthesis (or green synthesis) of silver nanoparticles is the use of eco-friendly and inexpensive organisms as a means to produce AgNPs. This approach has been the center of many researchers because it does not use some toxic chemicals in the process, and it can yield highly stable and well-characterized AgNPs6, 7.
In the synthesis of silver nanoparticles, especially in chemical methods, temperature is a crucial factor that affects the formation and growth of the particles8. In most cases, the reaction rate is heavily affected, which affects the morphology, size, and size distribution of the AgNPs. Determining the optimal temperature for the synthesis is then very important.
Silver nanoparticles are known to be unstable without stabilizing agents. Therefore, stabilizers play an important role in colloidal stability9. In addition, they also have a huge impact on the shape control, growth and antimicrobial activity of AgNPs9, 10, 11.
From the above bases, this study aims to provide potential chemically synthesized silver nanoparticles to be used as a disinfectant spray for pets. This solution helps deal with increasing skin problems in pets and offers another option to the existing imported products that are expensive. The use of only three component chemicals (sodium citrate, silver nitrate and polyvinyl alcohol) to fabricate the product (by reduction reaction) has made it much less expensive than other conventional products that do not incorporate AgNPs. Because only three chemicals were used, the study focused mainly on optimizing factors and conditions such as molar ratio and temperature to achieve the most prominent AgNPs. As a result, the silver nanoparticles in the product can effectively and rapidly eliminate most common bacteria found on pets’ skin and their environments without negatively affecting their health since they have suitable characteristics, such as relatively small size and low concentration. In addition, all the ingredients used in this product are orally and topically safe for pets.
METHODOLOGY
Market research
The very first step of making the product is to gain insight into the market demand. Therefore, we designed a research form that is used for investigating and collecting customer opinions about nanosilver disinfectant spray for pets. Specifically, we went directly to, or contacted remotely, pet stores and veterinary clinics in HCMC and performed an online survey via Facebook as follows:
Pet stores:
-
Pet Xinh (730 Lê Đức Thọ, Ward 15, Gò Vấp District, HCMC)
-
Miền Đất Hứa (367/8 Hoà Hảo, Ward 5, District 10, HCMC)
-
Nhà Vật Yêu (924 Nguyễn Trãi, Ward 14, District 5, HCMC)
-
Thế Giới Thú Cưng (424 Lạc Long Quân, Ward 5, District 11, HCMC)
-
Alice PetMart (7 Út Tịch, Ward 4, Tân Bình District, HCMC)
Veterinary clinics:
-
PetCare (146 Xóm Đất, Ward 9, District 11, HCMC)
-
New Pet Hospital & Spa (53 Đặng Dung, Tân Định Ward, District 1, HCMC)
-
Thithi (62B Hoà Bình, Ward 5, District 11, HCMC)
-
651 Vet Clinic (651/4 Điện Biên Phủ, Ward 1, District 3, HCMC)
Facebook groups/pages:
-
Đảo Chó (https://www.facebook.com/groups/kinhdoanhonline2104)
-
Đảo Mèo (https://www.facebook.com/groups/242848220423686)
These targets are classified into 2 groups: Group 1 consists of offline stores, and Group 2 consists of Facebook groups/pages. The survey was designed in such a way that we could have a good understanding of the market demand (Table 1). The data drawn from this survey provide an adequate amount of information for the implementation of the project. Simply put, according to the answers that the target customers give, we would know what to do or what to change to make the project more successful and ready.
List of questions for the survey
|
1 |
Do your pet(s) have skin problems? Yes No |
|
2 |
Do you often clean/wash your pet(s)? Often If necessary Rarely |
|
3 |
Have you ever used any disinfectant products for your pet(s). Please give the name of the product(s). |
|
4 |
If there was a disinfectant product for your pets (similar to hand sanitizer for humans), would you use it? Yes Maybe No |
|
5 |
If you could choose one type of the following products, which one would you choose? Good quality + No scent + Good price Good quality + Scent (green tea, mint, etc.) + Higher price |
|
The end. | |
Materials and fabrication process
The development of the product went through several stages. The purpose of this is to determine the best way to fabricate the product with the minimum use of chemicals and time while still maintaining a good product quality. Thereby, we can save time and money to increase the productivity and profit from the product.
The fabrication is based on a chemical approach to synthesize silver nanoparticles. In particular, a reduction reaction is used in this experiment with trisodium citrate (TSC) and silver nitrate (AgNO). The theoretical reaction is shown below:
4Ag + 1CHONa + 2HO → 4Ag + CHOH + 3Na + H + O
As seen in the reaction equation, TSC is used as a reducing agent to reduce Ag in AgNO into Ag (nanoparticles). However, there are many variations to execute this reaction. Many experiments must be performed to determine the best way to make AgNPs based on the above equation. In addition, for the nanoparticles to be stable over time, a stabilizing agent must be used. In this case, the stabilizer is polyvinyl alcohol (PVA).
Silver nitrate (AgNO) was purchased from Guangdong Guanghua Sei-Tech Co. Ltd. (China), trisodium citrate (NaCHO, 99.5%) from Fisher (USA), and polyvinyl alcohol (PVA) from Kuraray Poval™ (Singapore). Distilled water was made and provided by the Biomedical Engineering Laboratory, International University VNU. () ATCC 25923, () ATCC 25923, EMB agar and Baird-Parker agar were provided by Pasteur Institute, Ho Chi Minh city, Vietnam.
In this study, the chemical method was utilized for the synthesis of silver nanoparticles. In particular, the reduction reaction is the basis for the entire sequence of experiments in which trisodium citrate is the reducing agent that reduces Ag in silver nitrate into Ag. In addition, polyvinyl alcohol is the stabilizing agent. There are 4 stages that were performed in this fabrication process.
Stage 1: Optimization of the suitable procedure
First and foremost, we tested to see if putting AgNO or TSC first is better. The very first experiment was only about AgNO and TSC. At the beginning, two 20 mL vials were prepared. For the measurement to be convenient, we performed the experiments with 100 ppm output of AgNPs. In the first vial, we placed 3.15 mg of AgNO in 19.5 mL of distilled water and then heated it and stirred it at 70 degrees Celsius for 15 minutes. In the meantime, 1.36 mg of TSC was prepared in 0.5 mL of distilled water. After 15 minutes, 0.5 mL of TSC was added dropwise into a vial containing 19.5 mL of AgNO. The mixture was stirred until the color changed. In the second vial, the experiment was repeated similarly, but with TSC being added first, then AgNO was added later dropwise.
Stage 2: Evaluation of a sufficient molar ratio of AgNO:TSC
Theoretically, the molar ratio between AgNO and TSC is 4AgNO:1TSC, which means that 1 mole of TSC can reduce 4 moles of AgNO and yield 4 moles of Ag. However, the practical reaction efficiency is always lower than the theoretical efficiency. To examine which molar ratio between AgNO and TSC produces the highest reaction efficiency, we performed four trials in four 20 mL vials with four molar ratios – 4AgNO:1TSC, 1AgNO:1TSC, 1AgNO:4TSC and 1AgNO:16TSC. The method of making silver nanoparticles in this experiment is inherited from the conclusion in Stage 1, where AgNO should be added first. Specifically,
-
Vial 1 (4AgNO3:1TSC): 1.36 mg of TSC in 19.5 mL of distilled water + 3.15 mg of AgNO3 in 0.5 mL of distilled water.
-
Vial 2 (1AgNO3:1TSC): 5.45 mg of TSC in 19.5 mL of distilled water + 3.15 mg of AgNO3 in 0.5 mL of distilled water.
-
Vial 3 (1AgNO3:4TSC): 21.79 mg of TSC in 19.5 mL of distilled water + 3.15 mg of AgNO3 in 0.5 mL of distilled water.
-
Vial 4 (1AgNO3:16TSC): 87.16 mg of TSC in 19.5 mL of distilled water + 3.15 mg of AgNO3 in 0.5 mL of distilled water.
Stage 3: Evaluation of the effect of various PVA concentrations
PVA is commonly used as a stabilizing agent in nanoparticle synthesis. The role of it is to prevent the synthesized nanoparticles from aggregating or agglomerating together and then forming microparticles (micrometer size), which make them lose their properties as nanoparticles. The stabilizer accomplishes this advantage by capping the nanoparticles. Second, PVA can be used to control the growth of nanoparticles and their morphology. In this experiment, we used three different percentages of PVA (%w/v), 1%, 2% and 3%, to determine whether different amounts of PVA affect the quality of the synthesized AgNPs. This experiment is based on the results of the previous stage, which puts AgNO first and utilizes a molar ratio of 1AgNO:4TSC. First, we prepared three 19.5 mL solutions containing 1%, 2% and 3% PVA in three 20 mL vials. Then, the steps of the previous experiments were repeated, which involved adding TSC and then dropping AgNO.
Stage 4: Optimization of the adding PVA procedure
This experiment is once again inherited from the results obtained from the previous stage, which is adding TSC before adding AgNO, a molar ratio of AgNO:TSC of 1:4, and 2% PVA. Particularly,
-
Vial 1: 0.4 g PVA (2%) in 19.5 mL distilled water + 1.36 mg TSC + 3.15 mg AgNO3 in 0.5 mL distilled water.
-
Vial 2: 1.36 mg of TSC in 19.5 mL of distilled water + 3.15 mg of AgNO3 in 0.5 mL of distilled water + 0.4 g PVA (2%).
Absorption spectral measurement
The absorption spectrum of all the produced solutions during the entire fabrication process was measured using a Varioskan™ LUX multimode microplate reader (Thermo Scientific™) to examine the presence of the AgNPs within the range of absorption spectra from 300 – 700 nm and their general characteristics.
Hydrodynamic particle size measurement
The finalized product was brought into the Malvern Zetasizer Nano ZS (MAL1146785 – Malvern Panalytical) to measure its average hydrodynamic particle size and its size distribution to determine whether the product was accurately fabricated and whether the size of the particles is safe for pet use.
pH measurement
The finalized solution pH was measured by a Multiparameter pH-ORP-ISE-TEMP Bench Meter (AD1020 – Adwa) to evaluate whether the product is suitable for pet skin.
Antibacterial test
The final product was tested in vivo by Pasteur Institute Ho Chi Minh City, Vietnam using their standard IP HCM V04-2017 (BS EN 1040, BS EN 1500, ASTM E1054, ASTM E2315, BS EN 14347, BS EN 13727) in EMB and Baird Parker agar within one minute of exposure.
RESULTS
Market research data
Although the survey form was used to investigate all the targets, it is better and clearer to divide the results into two groups: Group 1 (Pet stores and veterinary clinics) and Group 2 (Facebook groups/pages).
There are a total of 9 data points collected from Group 1 and 322 data points collected from Group 2.
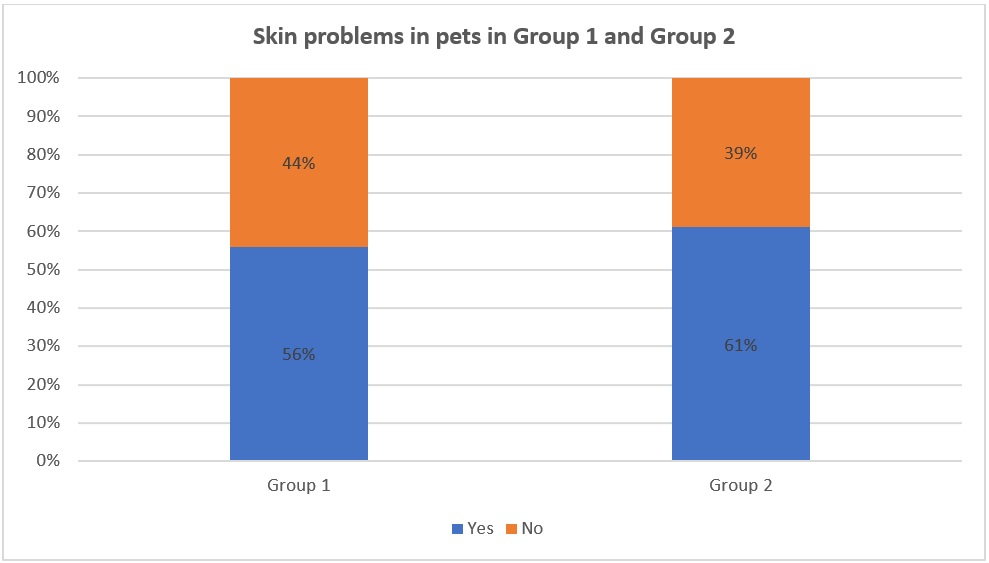
Data about skin problems in pets in Group 1 and Group 2

Frequency of cleaning/washing pets in Group 1 and Group 2

The willingness to use the disinfectant spray for pets in Group 1 and Group 2
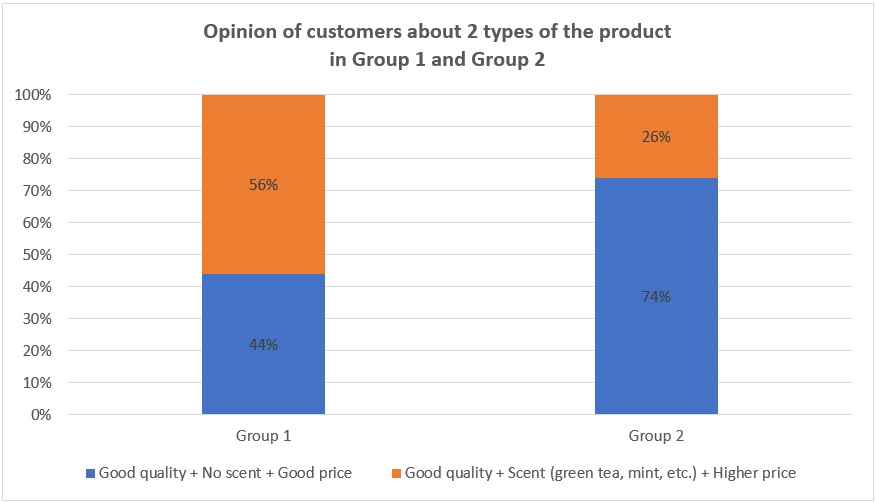
Opinion of customers approximately 2 types of products
results of the experiments in the fabrication process
All the mixtures obtained from all the experiments were measured in a UV–Vis spectrophotometer to observe their absorbance spectra.
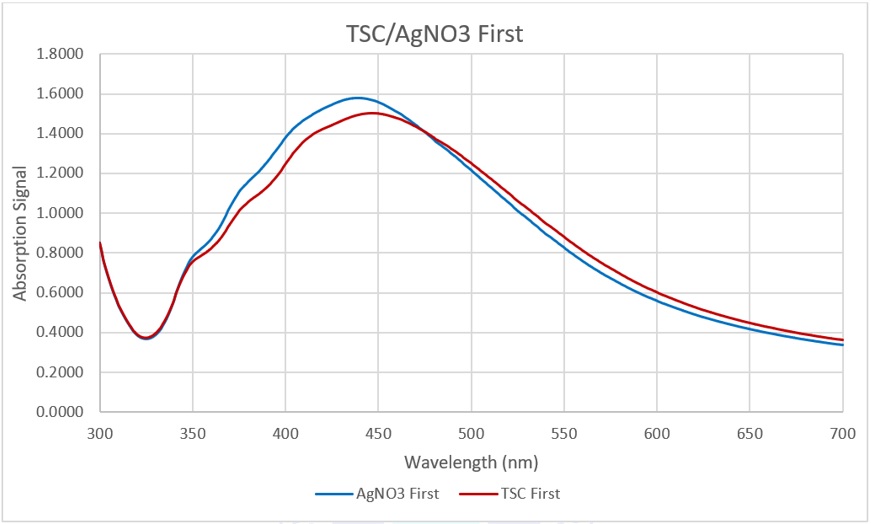
Absorbance spectra of the experiments in Stage 1

Absorbance spectra of the experiments in Stage 2

Absorbance spectra of the experiments in Stage 3

Absorbance spectra of 1% PVA and 2% PVA of the experiments in Stage 3 after 30 days
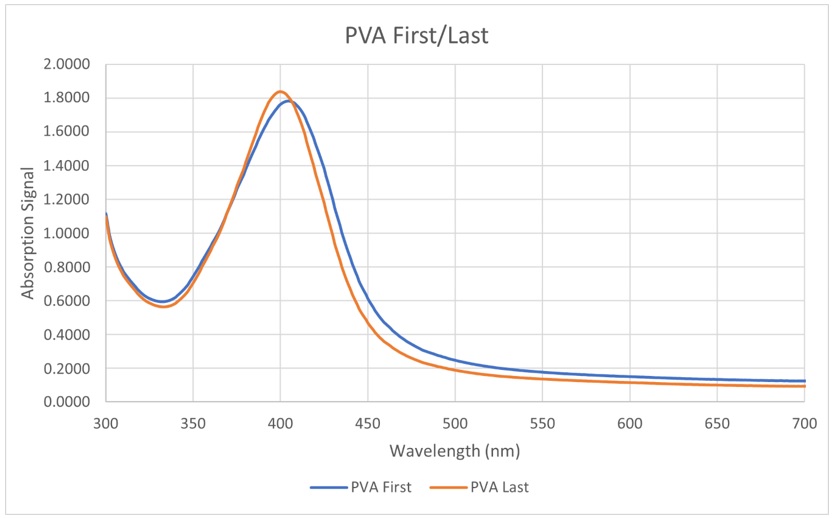
Absorbance spectra of the experiments in Stage 4
Finalization and characterization results
There are three things to be learned from the experiments: the order of using the chemicals, the molar ratio between AgNO and TSC, and the percentage of PVA. Specifically, PVA must be dissolved at the very beginning before adding TSC. Next, AgNO is added dropwise. Note that the mixture must be stirred continuously under high temperature (approximately 90-95°C) until the color changes then, the heating was stopped, and stirring was continued until room temperature. The percentage of PVA should be 2%, and the molar ratio of AgNO:TSC should be 1:4.
Scaling up to 1 liter of 30 ppm silver nanoparticle solution, we have the following official formula for the disinfectant spray for pets: First, we completely dissolved 20 g of PVA in 980 ml of distilled water at 90-95°C. After stirring and heating, 326.65 mg of TSC was added to the mixture until homogenous. While waiting for the mixture to be homogeneous, prepare a homogeneous solution of AgNO by adding 47.22 mg of AgNO to 20 ml of distilled water. Finally, aqueous AgNO was added dropwise to the mixture of TSC and PVA and stirred until the color changed to a greenish color, and then the heating was stopped. Stirring was continued until room temperature.
After fabricating the final product, a small sample extracted from it was brought into a Zetasizer to measure the hydrodynamic particle size of the AgNPs in the solution, which was approximately 49.79 nm on average (Figure 10).

Particle size of the final product using Zetasizer
In addition, the absorbance spectra of the final solution were observed over 3 months to evaluate its stability.

Absorbance spectra of the final product observed at 3 months
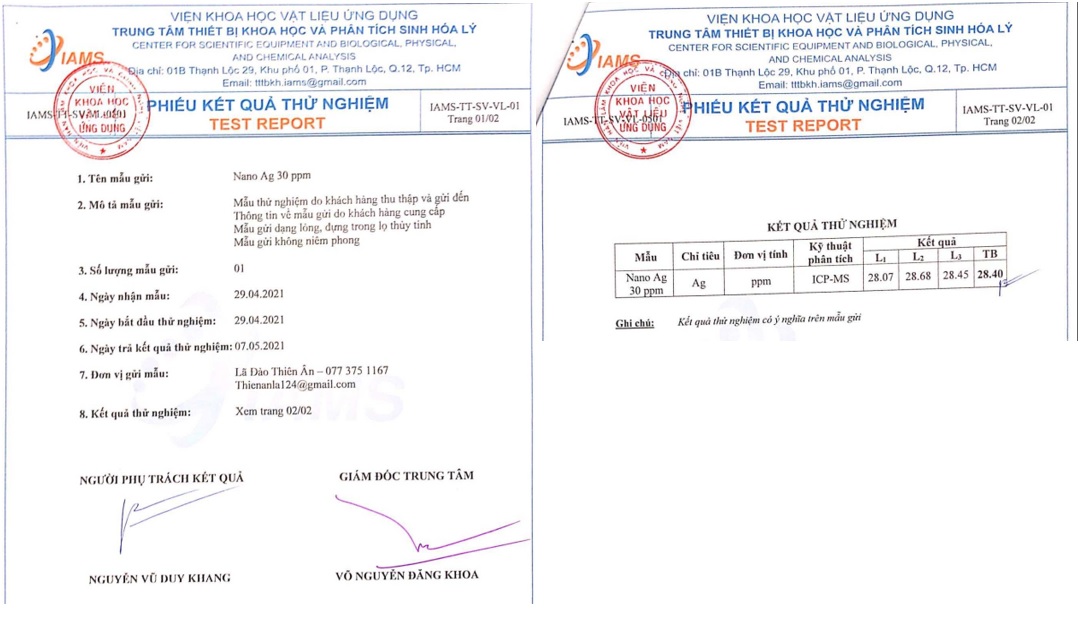
Next, to ensure that the approach to this formula is exact, the concentration of AgNPs must be measured using the ICP–MS technique. Theoretically, the formula is to make a 30 ppm AgNP solution, and the result shows that there are approximately 28.40 ppm AgNPs (Figure 13). Finally, the pH index was obtained using a pH meter, and the result was 6.35 (Figure 12).

pH of the produced solution
Antibacterial test result
The effectiveness of the product was examined by testing it on two bacteria: (gram-positive) and (gram-negative). Its effectiveness was 90.71% and on was 99.42% in one minute of exposure time (Figure 14).
DISCUSSION
Market research analysis
According to the charts in Figure 1, it can be seen that pets in both Group 1 and Group 2 that have skin problems accounted for over 50% (56% in Group 1 and 61% in Group 2). The difference in the percentage in Group 1 and Group 2 was not significantly different. This means that the situation of pets that have skin issues is quite common.
After asking people in Group 1 and Group 2 about how often they clean or wash their pets, the results are not surprising (Figure 2). Group 1 cleans and washes the pets much more often than Group 2 because pet stores and vet clinics must keep their pets clean and safe (78% often, and 22% if necessary). In Group 2, the owners do not have much time to clean or wash their pets frequently, as in pet stores or vet clinics. They only clean their pets when needed (51%). There are even 20% of them rarely washing their pets.
The charts in Figure 3 show the opinions of customers regarding whether they are willing to try the product as a disinfectant to keep their pets clean and safe. The results are very promising, as many people showed interest since there are 7 facilities (78%) that say Yes in Group 1 and 186 people (58%) that say Yes in Group 2. The percentage of those who are uncertain is 22% (2 facilities: Miền Đất Hứa and Thế Giới Thú Cưng) in Group 1 and 40% (129 people) in Group 2. Only 2% (7 people) say No in Group 2.
Finally, when asking people if they would rather choose an affordable product without scent or a product with scent but more expensive (Figure 4), 56% of them (most of them are in pet stores) in Group 1 choose the product with scent and higher price, and 44% choose the other one. The reason is that not only do they want their pets clean, but they also want their pets to have a good smell to attract buyers. In contrast, in Group 2, most people (237 out of 322) choose the nonscent product with a lower price (74%) since they do not care about the smell they care only whether their pets are clean and safe. In addition, they do not want to spend much money on such products. Only 26% of them desire the scented product.
The results reveal a very promising suggestion that the disinfectant spray for pets would be successfully implemented because the situation where pets have skin problems is visible. In addition, potential customers are willing to try this product to help their pets stay clean and safe without spending too much money and time. Last, the product that should be fabricated in the beginning is nonscent and low-price, while the quality has to be ensured.
Fabrication process analysis
The graph in Figure 5 shows that AgNPs are present in the mixtures in both vials. Nevertheless, in the vial where AgNO was added first, the peak of the signal was slightly higher than that of the vial with TSC first. These signals are not significantly different. By Stage 1, it is reasonable to put TSC first and then add AgNO later since adding TSC first can help make a stock solution containing only TSC without being afraid of its instability. This would make the sales safer and more cost-effective because when the orders come, the only task that needs to be done is adding AgNO, which is unstable in solution.
In Stage 2, with the same amount of silver nitrate in each vial, different amounts of trisodium citrate added gave different results (Figure 6). In the reaction where we used the theoretical molar ratio 4AgNO:1TSC, the signal was minimal. As seen in the next three data, the increase in TSC leads to the increase in signal. In other words, it appears that the theoretical molar ratio does not efficiently yield nanoparticles. The increase in TSC ensures that all Ag ions in the solution are reduced to make AgNPs. The molar ratios 1AgNO:4TSC and 1AgNO:16TSC provide the highest efficiency but are insignificantly different. In conclusion, the molar ratio of 1AgNO:4TSC is optimal (1AgNO:16TSC is unnecessary) to give the highest reaction efficiency.
Three solutions with three different amounts of PVA in Stage 3 were measured, and the results showed that there was no significance in the growth of AgNPs in all three mixtures (Figure 7). However, the solution with 3% PVA was rejected at the very beginning since its smell is quite uncomfortable, and its viscosity is quite high (~28 cP), which might irritate the pets. We only needed to consider 1% PVA versus 2% PVA. To decide which one is better, we left the two solutions for 30 days and measured their absorbance spectra again for evaluation. As a result, the graph (Figure 8) shows that the signal of 1% PVA changes. Specifically, its peak is lower and wider than before. This result suggests that the AgNPs in the solution containing 1% PVA aggregated. Hence, we chose 2% PVA because the AgNPs in the solution are more stable over time.
Finally, the absorbance spectra of the mixtures in Stage 4 were measured to decide whether we should add PVA at the beginning or at the end. It can be seen that the signals are similar (Figure 9). This means that putting PVA first or last does not truly affect the growth of the AgNPs. Nevertheless, we chose to add PVA first (before adding TSC) because we can make a stock solution out of PVA and TSC. Dissolving PVA is not too difficult however, mistakes can be made (not dissolved all, too much heat, etc.). This can significantly affect the entire solution if we add PVA at the end. Therefore, putting PVA at the very beginning is the best option.
Final product analysis
The average hydrodynamic particle size contained in the solution measured by Zetasizer is approximately 49.79 nm (Figure 10), which indicates good growth of AgNPs.
Next, according to the UV–Vis data (Figure 11), it is clear that the peaks are wider and lower over time (in 3 months). In other words, the data show that the produced AgNPs are stable over a long period of time. With the tendency of the graph, it can be induced that the product can be stored up to more than a year. Nevertheless, it is best to use it within one year.
Theoretically, the formula is to make a 30 ppm AgNP solution, and the result shows that there are approximately 28.40 ppm AgNPs. This means that the methodology of fabricating this product is accurate. The difference between the theoretical and practical AgNP concentrations possibly lies in the error in the fabrication process, such as the measurement of chemicals.
The product is safe for pet skin and edible. The solution has a pH of 6.35 (Figure 12), which is acceptable for pet skin since the acceptance range is approximately 5.5 – 712, 13. In addition, both the ingredients and the characteristics of the product are topically and orally safe for pets. Specifically, PVA is claimed to be edible and nontoxic, with LD(50)s in the range of 15-20 g/kg, and it does not accumulate in the body when pets lick and swallow it14, 15. In addition, PVA has been widely used in cosmetics and topical medicines, which means that PVA is nonirritating to the skin. The next ingredient that is used for synthesis is TSC, which is also a nontoxic chemical commonly used in the food and beverage industries and medicines16. TSC is approved by the US FDA (United States Food and Drug Administration) and USDA (United States Department of Agriculture) to be safe for oral and topical administration. Additionally. For all these reasons plus the characteristics of the product mentioned above, it can be said that this disinfectant spray is safe for pets when orally and topically administered, and it is effective in helping pets eliminate harmful bacteria.
Last, the in vitro testing result shows that the product can effectively kill bacteria such as (90.71%) and (99.42%) in just one minute of exposure (Figure 14).
CONCLUSION
Going through several stages of the experiments, a disinfectant spray for pets using chemically synthesized silver nanoparticles was successfully developed. As all the results show, the product is completely orally and topically safe for pets such as dogs, cats and rabbits since all the chemicals used for the fabrication are nontoxic. In addition, this disinfectant spray can kill gram-positive and gram-negative bacteria such as (90.71% in one minute) and (99.42% in one minute). These results confirm once again the safety and effectiveness of the product for pets. Meanwhile, the product can solve the current problem that the existing imported products are costly and inconvenient for daily use.
LIST OF ABBREVIATIONS
AgNPs: silver nanoparticles
TSC: Trisodium citrate
AgNO: silver nitrate
PVA: Polyvinyl alcohol
US FDA: United States Food and Drug Administration
USDA: United States Department of Agriculture
ICP–MS: Inductively coupled plasma–mass spectrometry
ACKNOWLEDGMENT
This research was funded by the Department of Science and Technology of Ho Chi Minh City under the grant number 01/2020/HĐ-QPTKHCN.
COMPETITING INTERESTS
The authors declare that they have no competing interests.
SUPPLEMENTARY
Concentration of AgNPs

In vitro testing of S.

