Synthesis and alpha-glucosidase inhibition of some novel quinazolin-4(3H)-one derivatives bearing a 2-thioxothiazolidine-4-one heterocycle
- Faculty of Pharmacy, Van Lang University, 233A Phan Van Tri Street, ward 11, Binh Thanh District, Ho Chi Minh City
- Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Street, Cau Giay District, Hanoi, Vietnam
- Ho Chi Minh City University of Technology, 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City
- Ho Chi Minh City University of Technology and Education, No 1, Vo Van Ngan Street, Linh Chieu Ward, Thu Duc City, Ho Chi Minh City
- Vo Van Tan high school, Section 3, Duc Hoa Town, Duc Hoa District, Long An Province
- Faculty of Chemistry, Ho Chi Minh City University of Education, 280 An Duong Vuong, Ward 4, District 5, Ho Chi Minh City
- Hau Nghia high school, Section A, Hau Nghia Town, Duc Hoa District, Long An Province
Abstract
Aimed at finding new compounds capable of inhibiting the enzyme glucosidase, six new N-(5-aryliden-4-oxo-2-thioxothiazolidin-3-yl)-2-{[4-oxo-3-(p-tolyl)-3,4-dihydro
quinazolin-2-yl]thio}acetamides 5a-f were synthesized by the reaction of N-(4-oxo-2-thioxothiazolidin-3-yl)-2-{[4-oxo-3-(p-tolyl)-3,4-dihydroquinazolin-2-yl]thio}acetamide 4 and appropriate aromatic aldehydes. The structural features of all synthesized compounds were confirmed based on their spectral data, including HR-MS, IR, 1H-NMR and 13C-NMR spectra. The results of the evaluation of the α-glucosidase enzyme inhibitory activity of acetamide compounds 5a-f showed that these compounds possess good inhibitor activity against the α-glucosidase enzyme, in which compound 5a containing the 4-methylbenzylidene moiety exhibited the best activity. Furthermore, molecular docking was conducted to better comprehend the binding properties of the compounds. The results indicated that synthesized quinazoline derivatives are potential antidiabetic compounds.
INTRODUCTION
According to the International Diabetes Federation, currently, between the ages of 20 and 79, 6.7 million adults will have diabetes, and three-quarters of people with diabetes will come from low-income countries1. Diabetes is a common disease and causes severe symptoms. Therefore, new drugs as well as new methods to treat this disease are being studied by many scientists.
The α-glucosidase enzyme is one of the causes of diabetes. Therefore, compounds with the ability to inhibit the α-glucosidase enzyme have the potential to become drugs for the treatment of diabetes. In addition to possessing some bioactivities, such as hypoglycemia2, 3, antimicrobial4, anticancer5, etc., hybrid compounds including quinazoline heterocycles incorporating thiazolidine heterocycles show good activity in the inhibition of the α-glucosidase enzyme, and the procedure to synthesize them is not very complicated6, 7, 8, 9. This work reported the synthesis and evaluation of the α-glucosidase enzyme inhibitory activity of -(5-aryliden-4-oxo-2-thioxothiazolidin-3-yl)-2-{[4-oxo-3-(-tolyl)-3,4-dihydroquinazolin-2-yl]thio}acetamide compounds as an extension of our study on the synthesis and bioactivity of compounds containing both quinazolin-4-one and thiazolidin-4-one heterocycles10.
MATERIALS AND METHODS
All chemicals (from Acros) and solvents (from Xilong) were utilized without any further purification.
Melting point determination of solid substances was performed by the capillary method with a Gallenkamp apparatus 5A-6797, which was uncorrected.
The IR spectra were measured on a Shimadzu FT-IR Affinity – 1S spectrometer using potassium bromide (KBr) pellets.
The H-NMR (500 MHz) and C-NMR (125 MHz) spectra were recorded on a Bruker Advance spectrometer using DMSO- as the solvent and internal standard. The chemical shift (δ) and coupling constants () are described in ppm and Hz, respectively. The abbreviations (singlet), (doublet), (double doublet), (triplet), (quartet), (multiplet), and (broad) represent NMR signal multiplicity.
Mass spectra were acquired from the Bruker micrOTOF-Q 10187.
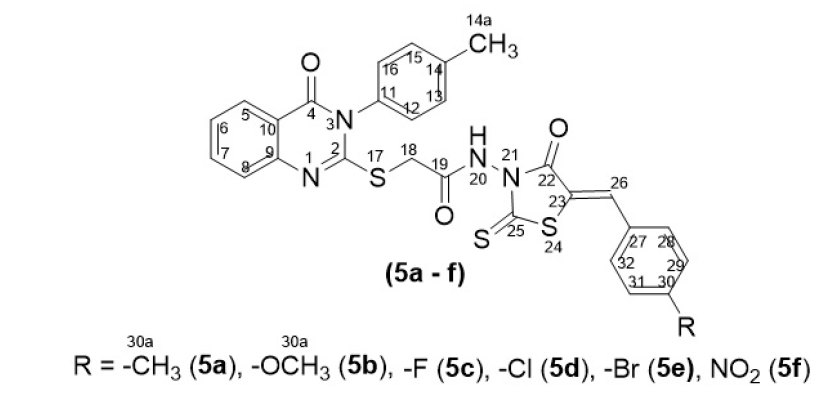
Compounds containing a 2-thioxothiazolidin-4-one nucleus incorporated into the 3-(-tolyl)quinazolin-4(3)-one heterocycle were produced following the synthetic sequences illustrated in Figure 1.
The examination of the enzyme α-glucosidase inhibitor activity was performed as described in11. The enzymes, substrates and sample solutions were established in 0.1 M potassium phosphate buffer (pH 7).
A reaction mixture of 50 µL each of α-glucosidase solution and serially diluted sample mixed with 100 µL buffer reagent was incubated at 37 °C for 20 minutes. Then, 50 µL of the substrate (pNPG) was added, and the obtained mixture was kept at 37 °C for another 30 minutes. The absorbance of the final solution was measured at a wavelength of 405 nm.
The formula aGI (%) = (A − B)/A × 100% was utilized to calculate the inhibition of the α-glucosidase enzyme, where aGI stands for α-glucosidase inhibitory activity, A is the absorbance of the mixture without a sample (inhibitor), and B is the absorbance of the mixture containing the sample. The inhibitor concentration required for 50% inhibition against α-glucosidase function under the assay conditions was defined as the IC value and determined by Table Curve AISN software.
Anthranilic acid (6.85 g, 0.05 mol) and potassium hydroxide (2.8 g, 0.05 mol) were dissolved in 60 mL of methanol. Carbon disulfide (9.5 g, 0.125 mol) and -toluidine (5.35 g, 0.05 mol) were slowly added to the mixture above and then refluxed for 10 hours. After pouring the reaction mixture into ice water, the precipitate was separated, dissolved in 10% potassium hydroxide and filtered to remove other solid impurities. Hydrochloric acid was added to the filtrate until the maximum precipitation. The pinkish precipitate was filtered and recrystallized from the mixture of DMF and HO to produce compound 1.
After stirring a mixture of 2-mercapto-3-(-tolyl)-quinazolin-4(3)-one (1) (2.68 g, 0.01 mol) and potassium carbonate (1.38 g, 0.01 mol) in DMF (50 mL) at room temperature for 30 minutes, ethyl chloroacetate (1.225 g, 0.01 mol) was added. The reaction mixture above was refluxed for 5 hours, cooled to room temperature and poured into ice water. Filtering of the white precipitate and recrystallization from ethanol afforded pure product 2.

Pathway for preparation of 2-thioxothiazolidin-4-ones derived from 2-mercapto-3-(p-tolyl)quinazolin-4(3
Hydrazine hydrate (NH. HO) (0.75 g, 0.015 mol) was slowly added dropwise to a solution of ester 2 (3.54 g, 0.01 mol) dissolved in 50 mL of ethanol. After refluxing for 5 hours, the reaction mixture was kept overnight at room temperature. The solid formed was filtered and recrystallized from ethanol to yield compound 3.
Thiocarbonyl--thioglycolic acid (1.469 g, 6.5 mmol) was added to a solution of compound 3 (2.04 g, 6 mmol) dissolved in 15 mL of absolute ethanol. The mixture was refluxed for 8 hours and then cooled down. The precipitate was filtered off and recrystallized from acetic acid to give the yellowish powder compound 4.
Compound 4 (0.456 g, 1 mmol) was dissolved in 15 mL of glacial acetic acid, and then the appropriate aldehyde (1 mmol) and CHCOONa (0.082 g, 1 mmol) were added. After refluxing for 5 hours, the reaction mixture was cooled down, poured into ice water and filtered for solid. Recrystallization from a proper solvent produced pure substances 5a-f.
The physical properties and IR spectral data of compounds 5a-f are shown in
The physical properties and IR spectral data of the compounds 5a-f (structure was shown in
|
Comp. |
Solvent recrystallized |
Mp. (oC) |
Yield (%) |
ν (cm-1) | ||||
|
N-H |
C-H aromatic |
C-H aliphatic |
C=O |
C=N, C=C | ||||
|
5a |
AcOH : H2O |
215 |
75 |
3192 |
3011 |
2916 |
1744, 1690 |
1593, 1549, 1466 |
|
5b |
AcOH : H2O |
223 |
78 |
3198 |
3009 |
2924 |
1744, 1688 |
1580, 1551, 1468 |
|
5c |
AcOH : H2O |
233 |
70 |
3381 |
3089 |
2980 |
1739, 1688 |
1651, 1579, 1468 |
|
5d |
AcOH : H2O |
238 |
73 |
3200 |
3013 |
2918 |
1740, 1688 |
1603, 1579, 1466 |
|
5e |
AcOH : H2O |
253 |
71 |
3190 |
3010 |
2956 |
1743, 1688 |
1594, 1550, 1468 |
|
5f |
AcOH : H2O |
235 |
77 |
3185 |
3005 |
2938 |
1742, 1686 |
1599, 1552, 1468 |
Docking study
The ligand‒protein interactions were investigated by utilizing AutoDock Vina software (version 1.1.2) from the Scripps Research Institute, San Diego, California, USA. The target protein data, -glucosidase [PDB: 5KWZ], originated from the RCSB Protein Data Bank. Both the -glucosidase and the studied compounds were prepared properly before performing the docking method. Based on the native ligand, -glucosidase was prepared, and its active site was also identified via Autodock Tool software version 1.5.6. The geometrical center was determined and illustrated in a three-dimensional grid with a size of 40 Å 40 Å 40 Å, in which X = 2.5, Y = -19.2 and Z = 25.2. Subsequently, all the compounds were energy minimized by Avogadro Version 1.2.0. Visualization and evaluation of the outcomes for postdocking analysis were performed via Discovery Studio 2021 Client.
RESULTS
Synthesis and structural study
light-pink crystal (10.05 g), yield 75%, mp: 170 °C (7: 169 – 171 °C). IR (, cm): 3242, 3122 (N-H, S-H), 3028 (C-H aromatic), 1659 (C=O), 1620, 1522, 1485 (C=N, C=C aromatic); H-NMR (, ppm): 13.01 (1H, , SH), 7.96 (1H, , 8.0 Hz, Ar-H), 7.79 (1H, , 8.0 Hz, Ar-H), 7.45 (1H, , 8.5 Hz, Ar-H), 7.35 (1H, , 7.5 Hz, Ar-H), 7.28 (2H, , 8.0 Hz, Ar-H), 7.15 (2H, , 8.0 Hz, Ar-H), 2.38 (3H, , -CH); C-NMR (, ppm): 176.7 (C=O), 160.3, 140.1, 137.9, 137.2, 136.0, 129.9, 129.2, 127.9, 124.8, 116.7, 116.2 (C), 21.3 (CH).
white crystal (2.478 g), yield 70%, mp: 101 – 102 °C (12: 102 °C). IR (, cm): 3061 (C-H aromatic), 2986, 2914 (C-H aliphatic), 1728 (C=O ester), 1694 (C=O ketone), 1606, 1547, 1466 (C=N, C=C aromatic); H-NMR (, ppm): 8.08 (1H, , 8.0 Hz, Ar-H), 7.82 (1H, , 8.0 Hz, Ar-H), 7.49 (2H, , Ar-H), 7.40 (2H, , 8.0 Hz, Ar-H), 7.34 (2H, , 8.0 Hz, Ar-H), 4.15 (2H, , 7.0 Hz, -CHCH), 3.98 (2H, , -SCH-), 2.43 (3H, , -CH), 1.22 (3H, , 7.0 Hz, -CHCH); C-NMR (, ppm): 168.8, 161.1 (C=O), 157.2, 147.5, 140.3, 135.4, 133.5, 130.6, 129.5, 127.1, 126.6, 126.3, 119.9 (C), 61.5 (-OCHCH), 34.9 (-SCH), 21.3 (-CH), 14.6 (-OCHCH).
: white crystal (2.176 g), yield 64%, mp: 146 – 148 °C (12: 148 °C). IR (, cm): 3271 (N-H), 3062 (C-H aromatic), 2974 (C-H aliphatic), 1678, 1643 (C=O), 1606, 1548, 1468 (C=N, C=C aromatic); H-NMR (, ppm): 9.30 (1H, , NH), 8.09 (1H, , 8.0 Hz, Ar-H), 7.85 (1H, , 8.0 Hz, Ar-H), 7.63 (1H, , 8.0 Hz, Ar-H), 7.49 (1H, , = 8.0 Hz, Ar-H), 7.39 (2H, , 8.0 Hz, Ar-H), 7.34 (2H, , 8.0 Hz, Ar-H), 4.27 (2H, , NH), 3.84 (2H, , SCH), 2.50 (3H, , CH). C-NMR (, ppm): 166.6, 161.2 (C=O), 157.5, 147.6, 140.1, 135.3, 133.6, 130.5, 129.6, 127.0, 126.6, 126.5, 120.0 (C), 35.0 (-SCH-), 21.3 (-CH).
2-Mercapto-3-(-tolyl)-quinazolin-4(3)-one 1, ethyl 2-{[4-oxo-3-(-tolyl)-3,4-dihydroquinazolin-2-yl]thio}acetate 2, and 2-{[4-oxo-3-(-tolyl)-3,4-dihydroquinazolin-2-yl]thio}acetohydrazide 3 were synthesized according to4, 6, 7, 12. The matching of both melting point and spectral data provides evidence that these compounds were successfully synthesized.
white crystal (1.97 g), 72% yield, mp: 246 °C. IR (, cm): 3210 (NH), 3094 (C-H aromatic), 2978 (C-H aliphatic), 1761, 1688 (C=O), 1609, 1549, 1466 (C=N, C=C aromatic); H-NMR (, ppm): 11.28 (1H, , NH), 8.09 (1H, , 8.0 Hz, Ar-H), 7.85 (1H, , 8.0 Hz, Ar-H), 7.75 (1H, , 8.0 Hz, Ar-H), 7.49 (1H, , = 8.0 Hz, Ar-H), 7.40 (2H, , 8.0 Hz, Ar-H), 7.34 (2H, , 8.0 Hz, Ar-H), 4.41 (2H, , CH thiazolidine), 4.13 (2H, , -SCHCONH-), 2.43 (3H, , CH); C-NMR (, ppm): 200.1 (C=S), 170.5, 166.0, 161.2 (C=O), 156.8, 147.6, 140.3, 135.2, 133.5, 130.5, 129.6, 126.9, 126.5, 120.0 (C), 66.8 (C=N), 34.5 (-S-CH-), 33.8 (CH thiazolidine), 21.3 (CH); HR-ESI-MS 479.0275 (M + Na), calc. for CHNNaOS 479.0277.
The synthesis results and spectral data of compounds 5a-f are shown in

The structure of compounds 5a-f
1H-NMR (δ, ppm and
|
Signal |
R = -CH3 (5a) |
R = -OCH3 (5b) |
R = -F (5c) |
R = -Cl (5d) |
R = -Br (5e) |
R = -NO2 (5f) |
|
H5 |
8.09 ( |
8.10 ( |
8.09 ( |
8.09 ( |
8.10 ( |
8.10 ( |
|
H6 |
7.89 ( |
7.89 ( |
7.88 ( |
7.88 ( |
7.88 ( |
7.88 ( |
|
H7 |
7.50 ( |
7.50 ( |
7.50 ( |
7.50 ( |
7.50 ( |
7.51 ( |
|
H8 |
7.78 ( |
7.78 ( |
7.78 ( |
7.78 ( |
7.79 ( |
7.78 ( |
|
H12, H16 |
7.40 ( |
7.41 ( |
7.41 ( |
7.41 ( |
7.41 ( |
7.41 ( |
|
H13, H15 |
7.35 ( |
7.35 ( |
7.35 ( |
7.35 ( |
7.35 ( |
7.35 ( |
|
H14a |
2.43 ( |
2.43 ( |
2.43 ( |
2.43 ( |
2.44 ( |
2.43 ( |
|
H18 |
4.16 ( |
4.16 ( |
4.16 ( |
4.16 ( |
4.16 ( |
4.16 ( |
|
H20 |
11.51 ( |
11.50 ( |
11.54 ( |
11.55 ( |
11.54 ( |
11.59 ( |
|
H26 |
7.88 ( |
7.88 ( |
7.94 ( |
7.93 ( |
7.91 ( |
8.04 ( |
|
H28, H32 |
7.58 ( |
7.67 ( |
7.78 ( |
7.77 ( |
7.79 ( |
8.37 ( |
|
H29, H31 |
7.39 ( |
7.15 ( |
7.43 ( |
7.64 ( |
7.64 ( |
7.95 ( |
|
H30a |
2.38 ( |
3.86 ( |
- |
- |
- |
- |
13C-NMR (δ, ppm) spectral data of compounds 5a-f (see structure in
|
Comp. |
| |||||
|
R |
C=S |
C=O |
Csp2 |
CH2 |
CH3 | |
|
5a |
-CH3 21.3 |
190.5 |
166.2, 163.6, 161.2 |
156.8, 147.6, 142.5, 140.3, 135.3, 135.2, 133.5, 131.5, 130.7, 130.6, 130.5, 129.6, 127.0, 126.6, 120.0, 118.5 |
34.5 |
21.6 |
|
5b |
-OCH3 56.1 |
190.5 |
166.2, 163.6, 162.3 |
161.2, 156.8, 147.6, 140.3, 135.3, 135.2, 133.7, 133.5, 130.5, 129.6, 127.0, 126.6, 125.8, 120.0, 116.3, 115.7 |
34.5 |
21.3 |
|
5c |
-F |
190.4 |
166.3; 164.9; 163.5 |
156.8, 147.6, 140.3, 135.2, 134.1, 134.0, 133.9, 133.5, 130.6, 130.0, 129.9, 129.6, 127.0, 126.6, 120.0, 119.5 |
34.5 |
21.3 |
|
5d |
-Cl |
190.3 |
166.3; 163.5; 161.2 |
156.8, 147.6, 140.3, 136.5, 135.2, 133.8, 133.5, 133.0, 132.1, 130.6, 130.1, 129.6, 127.0, 126.6, 120.5, 120.0 |
34.5 |
21.3 |
|
5e |
-Br |
190.2 |
166.3; 163.5; 161.2 |
156.8, 147.6, 140.3, 135.2, 133.9, 133.5, 133.1, 132.4, 130.6, 129.6, 128.7, 127.0, 126.6, 125.5, 120.6, 120.0 |
31.2 |
21.3 |
|
5f |
-NO2 |
190.0 |
166.3; 163.3; 161.2 |
156.8, 148.4, 147.7, 140.3, 139.2, 135.2, 133.5, 132.3, 132.1, 130.6, 129.6, 127.0, 126.6, 124.9, 124.1, 120.0 |
34.5 |
21.3 |
HR-ESI-MS spectral data of compounds 5a-f
|
Comp. |
Molecular formula |
[M+H]+ calcd. |
[M+H]+ |
|
5a |
C28H22N4O3S3 |
C28H22N4O3S3+H 559.0927 |
559.0934 |
|
5b |
C28H22N4O4S3 |
C28H22N4O4S3+H 575.0876 |
575.0884 |
|
5c |
C27H19FN4O3S3 |
C27H19FN4O3S3+H 563.0682 |
563.0695 |
|
5d |
C27H19ClN4O3S3 |
C27H19ClN4O3S3+H 579.0386 |
579.0379 |
|
5e |
C27H19BrN4O3S3 |
C27H19BrN4O3S3+H 622.9875 |
622.9883 |
|
5f |
C27H19N5O5S3 |
C27H19N5O5S3+H 590.0621 |
590.0646 |
Enzyme α-glucosidase inhibition activity
-Glucosidase is an important enzyme in the hydrolysis of carbohydrates to form glucose. Therefore, the compounds that had -glucosidase inhibition can slow the absorption of glucose, which is effective in diabetes type 2 treatment.
Enzyme -glucosidase inhibition activity results (see
Molecular docking, as an approach, is used to study the interaction between receptor (protein) targets and small compounds (ligands) 13. The docking score, which is also known as the binding energy obtained from docking, is regarded as a function of the binding affinity of the ligand to the protein target 13. This study modeled synthesized compounds to determine their inhibitory activity toward the enzyme α-glucosidase through molecular docking. The docking results and IC values of these compounds are shown in
Enzyme
|
Compounds |
IC50 (µg mL-1) |
Docking score (kcal mol-1) |
H-bonds |
|
Acarbose |
235.36 ± 3.08 |
- |
- |
|
4 |
- |
-7.8 |
TRP126, ALA93 |
|
5a |
15.07 ± 0.97 |
-8.8 |
GLY123, TRP126, ALA93 |
|
5b |
15.11 ± 1.94 |
-8.6 |
TRP126, ALA93 |
|
5c |
71.17±2.22 |
-8.8 |
TRP126, ALA93 |
|
5d |
15.15±0.15 |
-8.5 |
TRP126, ALA93 |
|
5e |
95.42 ± 1.88 |
-8.9 |
TRP126, ALA93 |
|
5f |
20.44 ± 2.64 |
-8.6 |
TRP126, ALA93 |
DISCUSSION
The synthesis of 4 starting from anthranilic acid was reported in our previous work 14. Compound 4 bearing a 2-thioxothiazolidin-4-one moiety in which an active methylene group was reacted with aromatic aldehydes via Knoevenagel condensation to form -(5-aryliden-4-oxo-2-thioxothiazolidin-3-yl)-2-{[4-oxo-3-(-tolyl)-3,4-dihydroquinazolin-2-yl]thio}acetamides 5a-f, respectively (see the physical properties in
In compounds 5a-f, the carbonyl group of the thioxothiazolidin-4-one ring joins the conjugation system with the benzylidene moiety therefore, its signal in the IR spectra (see
In comparison with the H-NMR spectrum of compound 4, the signal of the methylene protons in the thiazolidine heterocycle at 4.41 ppm did not appear in compounds 5a-f, whereas a new singlet signal attributed to the methine proton (H) was found at approximately 7.88-8.04 ppm in the spectra. Additionally, the methylene group peak outside the thiazolidine ring (-SCHCONH-) and the aryl proton peak also appeared as predicted. All signals in C-NMR (see
Compound 4, with an unsubstituted 5 position of the thiazolidine ring, did not show bioactivity against α-glucosidase. Six compounds, 5a-f (

2D representation of ligands 5a (a), 5b (c), 5c (e), 5d (g), 5e (i), 5f (k), 4 (m) and 3D representation of ligands 5a (b), 5b (d), 5c (f), 5d (h), 5e (j), 5f (l), 4 (n) docked to α-glucosidase.
Despite the excellent outcome with IC, the docking score of 5a is the second top scorer. This compound has a hydrogen bond acceptor at N1 of the quinazoline ring with TRP126, a van der Waals interaction with GLY123, and a hydrogen bond donor at the amide nitrogen with ALA93 (Figure 3a).
Three compounds, 5b, 5d and 5f, show similarities between IC and docking score. Compound 5b has a hydrogen bond acceptor (Figure 3c) at N1 of quinazoline with TRP126 and a hydrogen bond donor at the amide nitrogen with ALA93 and a pi-cation interaction between benzene, which has a methoxy group, and ARG275; moreover, there is an alkyl interaction of the -CH group with VAL544. Compound 5d has a hydrogen bond acceptor at N1 of quinazoline with TRP126 and a hydrogen bond donor with ALA93 at N of the amide functional group (Figure 3g). Furthermore, this compound has a hydrogen bond with ASP91 and many van der Waals interactions. Compound 5f has a hydrogen bond acceptor at N1 of quinazoline with TRP126 and a hydrogen bond donor with ALA93 at N of the amide functional group (Figure 1k).
Compounds 5e (Figure 3i) and 5c (Figure 3e) with relatively high IC values (95.42 ± 1.88 µg mL and 71.17 ± 2.22 µg mL, respectively) do not show good activity, but they have the best docking score (
Hydrogen bond interactions with the binding site and the catalytic residues of an enzyme significantly affect the activity of the target proteins and result in inhibitory modulation of their training15. The findings in this study show several hydrogen bonds interacting with the residues of the receptor binding site. The residues residing out of the binding site ranged from two to four conventional hydrogen bond interactions. Hydrogen bond interactions of residues outside an enzyme's active site area can affect the target receptor's activity by disordering the polypeptides that constitute the active site, as mentioned by William ., 2012 16 in their study with myricetin and ethyl caffeate.
Noncovalent binding interactions such as Pi-pi stacking, Pi-sigma stacking, and alkyl stacking are crucial for exacting necessary biological activities during ligand‒protein interactions15, 17. Compound 5a, having the best IC activity, has shown noncovalent interactions (Figure 3a) with the receptor under study, which might support its activity.
CONCLUSIONS
By a simple and convenient method, compound 4 and six 5-arylidene derivatives were successfully obtained. Their chemical structures were elucidated by the typical analysis of IR, NMR and HR-MS spectral data. Compounds 4 and 5a-f were evaluated for α-glucosidase inhibition activity, and compounds with an electron donating group in the benzylidene moiety showed good activity, especially compound 5a containing the 4-methylbenzylidene moiety, which exhibited the strongest activity among them. The results of molecular docking, including docking score and interactions, prove that quinazoline derivatives are potential antidiabetic compounds.
LIST OF ABBREVIATIONS
C-NMR: Carbon-13 nuclear magnetic resonance
DMSO: dimethyl sulfoxide
H-NMR: Proton nuclear magnetic resonance
HR-MS: High-resolution mass spectrometry
IR: Infrared spectroscopy
IC: Half-maximal inhibitory concentration
pNPG: 4-Nitrophenyl-α-D-glucopyranoside
COMPETING INTERESTS
The authors declare that there are no competing interests or any personal problems that could affect this work reported.
Authors' Contributions
Bui Thi Thuy Linh: conceptualization, methodology, investigation, writing – original draft preparation; Nguyen Nguyen Cuong Phat, Nguyen Tuan Kiet: investigation; Nguyen Thi Kim Yen: revewing and editing; Nguyen Tien Cong: conceptualization, supervision; Le Trong Duc: analysis, writing – revewing and editing. All authors have read and agreed to the published version of the manuscript.

