Synthesis of a novel polysulfide via the reaction of a thiol compound and oxidant towards polymer self-healing application
- National Key Laboratory of Polymer and Composite Materials– Ho Chi Minh City, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam
- Vietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc City, Ho Chi Minh City, Viet Nam
- Faculty of Materials Technology, Ho Chi Minh City University of Technology, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam
Abstract
Introduction: This legion is an indiscernible virtue of polysulfide that it has been employed in a range of fields. In addition, the oxidation of thiol to reversible disulfide is a dramatically preponderant process in biology and chemistry, especially the production of healable polymers. Interestingly, thiol is widely utilized to synthesize numerous polymers through the thiol-click reaction. From the sound of it, our underlying intent in this research is the synthesis of thiol-ended polysulfide potential for the self-healing polymer.
Method: In this study, the synthesis of polysulfide carrying the thiol terminal group was undertaken out of the oxidizing reaction in the presence of iodine. Its reaction time was monitored by means of TLC. Furthermore, state-of-the-art measurements of FT-IR, 1H-NMR and GPC were used to represent a great deal of valuable information pertaining to its structure and molecular weight.
Result: After a period of 24 hours at room temperature, the reaction producing pure polysulfide was completed. The FT-IR and 1H-NMR results show that the polymer was successfully synthesized; moreover, its Mn is approximately 12000 g/mol.
Conclusion: This study will play a vital role in facilitating the use of thiol-terminated polysulfide in the synthesis of self-healing materials in the not-too-distant future.
INTRODUCTION
Polysulfide can be viewed as a type of polymer bearing atoms of sulfur in its molecular chains 1. Polysulfide possesses a profusion of incomparable merits, such as showing superb properties at low temperatures2, being stable in a wide assortment of solvents and water3, 4 and the ability to stick to catholic surfaces5, and has been applied in numerous industrial applications ranging from adhesives6, sealants7 and coatings8, 9. In addition, the disulfide-bond-owning polymer can be used to synthesize self-healing materials out of the occurrence of S‒S links in its main chains10, 11, 12. These linkages are likely to engage in the self-healing process because their reversible quality allows them to repeatedly break and connect under various conditions of UV light and heat with a want of catalysts 13, 14, 15. It is imperative that the synthesis of polysulfide is of great importance in doing wonders for the prevalence of versatile polymers in different fields.
In regard to the synthesis of polymers, click-thiol reactions count as a viable tool in view of their high reaction yield, the elimination of byproducts void of the implementation of chromatographic methods, being insensitive to oxygen and water and simple reaction conditions with a lack of the addition of solvents. There is a wide assortment of these reactions, such as thiol-ene, thiol-yne, thiol-isocyanate, thiol-epoxy, and thiol-bromo reactions16.
On the one hand, it is unequivocal that utilizing a number of catalysts with metal atoms in their structure, such as Fe, Pb, Ce, Mn, Co, Cr, Cu and Al, to oxidize thiol groups can be seen as a common means of forming disulfide links17, 18. It is odds-on that disulfide groups react with strong oxidizing agents to create thiosulfinate và thiosulfinate ester; having said that, this reaction is extremely slower than that of thiol, signifying that only at high temperatures can it occur 19. The oxidizing process of thiol groups, which are ascribed to hydrogen peroxide and alkyl hydroperoxide together with peroxyacid, produces a primary product, disulfide. However, it is likely to be oxidized by residual oxidants with ease.
On the other hand, oxidizing agents such as halogen (I, Br), hydrogen peroxide, DDQ and sodium nitrite are harnessed in this process 20, 21. Recently, a plethora of research reported the oxidization of thiol groups out of visible light as well as photocatalysts such as diaryl telluride 22 and iron phthalocyanine fixed to graphene 23.
After the oxidization of thiol and halogen, the formation of different products is reliant on the type of halogen and conditions of the reaction. In water, the reaction between chlorine, bromine and thiol gives rise to the emergence of sulfonyl halide or sulfonic acid. On the flip side, without water, halogen derivative disulfide compounds can be obtained. Iodine can be deemed the most prevalent agent catering for thiol diverted to disulfide18. Interestingly, it can take place under manifold conditions, such as inert solvents, ethanol, pentane and acetonitrile, with not only no need for the addition of other agents24 but also several agents, such as triethylamine or cerium(III) chloride heptahydrate25.
In this study, our objective in this paper is to synthesize polysulfide via the reaction of a thiol compound and the oxidant I. Thus, 2,2’-(Ethylenedioxy)diethanethiol was oxidized by I(Figure 1), and disulfide groups were established in the structure of the polymer. In addition, it has flexible thiol groups at the end of its chain. They can enable this polymer to connect with other polymers for synthesizing self-healing materials. Subsequently, through results from the FT-IR, GPC, TLC and H-NMR methods, the polymer was confirmed to be prepared in a successful manner.

Synthesizing the polysulfide from the reaction of 2,2’-(ethylenedioxy)diethanethiol and I2 at room temperature
EXPERIMATION
Materials
2,2’-(Ethylenedioxy)diethanethiol (95%, Sigma‒Aldrich), iodine (Fisher), trichloroacetyl isocyanate (TAI, Acros), potassium carbonate (UNI-chem), dimethylformamide, chloroform (Fisher)
Characterization
H-NMR spectra were obtained using a Bruker Advance 500 MHz spectrometer; the solvent used was CDCl with tetramethylsilane as an internal reference. Transmission Fourier transform infrared (FT-IR) spectra were recorded from KBr disks by an FT-IR Bruker Tensor 27 with an average of 256 scans and a resolution of 4 cm. GPC measurements were carried out using a Polymer PL-GPC 50 gel permeation chromatograph system equipped with an RI detector and THF as the eluent at a flow rate of 1.0 mL/min. With reference to polystyrene standards, the molecular weight and molecular weight distribution (D) were computed. Thin layer chromatography (TLC) plates were purchased from Sigma‒Aldrich.
Synthesis of polysulfide
First, the thiol compound and the oxidizing agent were dissolved in DMF solvent. Afterwards, the thiol solution was added to the reaction vial and stirred. Then, the iodine solution (20 wt%) was slowly pipetted into the reaction glass prior to being mixed together at room temperature over the given timescales – 6 h and 24 h. The progress of the reaction was checked via thin layer chromatography (TLC). After that, the mixture was washed with a saline solution of NaSO (10 wt%) to totally eliminate iodine and part of DMF. Next, it was added to CHCl3 to produce organic components before being washed with water (2-3 times) to completely eradicate DMF. In the following step, the mixture was extracted to attain the organic phase soluble in CHCl3 and entirely anhydrous with KCO absorbing the remaining water. Finally, the mixture was evaporated to collect the product. DI06 and DI24 were named two polysulfide products obtained after 6 h and 24 h, respectively.
RESULTS
Polysulfide was acquired according to the process depicted in Figure 1, and its weight yield is displayed in

TLC results (hexane/ethyl acetate = 5/1 (v/v)) of dithiol (green), DI24 (red) and DI24 (purple)
GPC results of DI06 and DI24 after the reactions over the periods of 6 h and 24 h
|
Ratio |
React time |
Product |
Mn (GPC) |
IPD |
Yield | |
|
Dithiol |
2:1 |
6 h |
DI06 |
20.863 |
1.4 |
68,97% |
|
Dithiol |
2:1 |
24 h |
DI24 |
28.768 |
1.1 |
55,80% |

GPC spectra (THF, 1.0 mL/min) of DI06 and DI24 in a room after 6 h and 24 h
The polymer's molecular weight is illustrated in the GPC curves (Figure 3), and related information is given in

FT-IR spectra (4000-500 cm-1, 256 scans) of dithiol (bottom), DI06 (middle) and DI24 (top) after the synthesis process.
Regarding results from the FT-IR method, Dithiol, DI06 and DI24 register their peaks at 2864 cm, 1352 cm, 1294 cm and 1112 cm attributed to the stretching and bending of the sp CH group, the C-S bond and alkoxy group correspondingly. It is easy to observe that only dithiol exhibits a band located at 2557 cm. The reason behind this is the thiol group stretching.

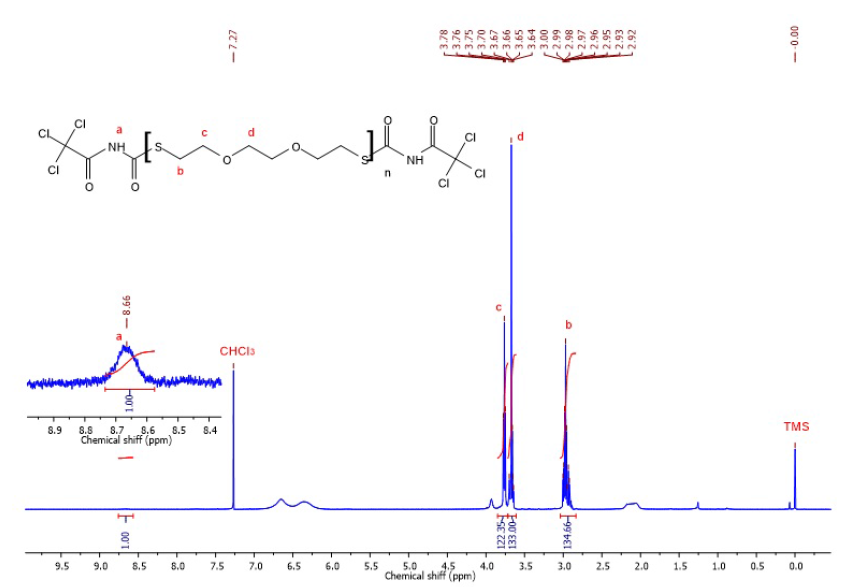
1H-NMR (500 MHz, CDCl3) spectrum of polydithiol DI24 reacting with trichloroacetyl isocyanate (TAI).
The structure of DI24 was characterized via H-NMR. Herein, DI24 reacted with TAI to transform the thiol proton at the end of its chain to the proton of imide. As a result, the peak (a) at 8.66 ppm is attributable to the imide’s proton; therefore, there is still the appearance of thiol groups. In addition, other peaks (b, c, d) are in line with those of the structure of polysulfide. In attempts to determine the molecular weight of the polymer, the comparison between the integral area of the CH proton of the imide group (the a peak) and the CH proton of the polysulfide (the b peak) was carried out by formulas illustrated below:
where is the number of repeating units of polysulfide. Then, we have
Next, it is clear that
Consequently, the value is approximately 12214 g/mol.
DISCUSSION
It is the formation of polysulfide that was conducted at room temperature with the assistance of I, an oxidizing agent; 2,2’-(ethylenedioxy)diethanethiol is viewed as the dithiol monomer, which was oxidized to form polysulfide with the presence of thiol groups at the end of its chain (Figure 1) 25. The reaction took place in DMF with a mole ratio of thiol groups and I of 2:1. Moreover, the oxidation of thiol with the assistance of I took place over two different periods, 6 h and 24 h, which are analogous to those of previous studies24, 25.
According to the TLC results of DI06 and DI24, there was a transformation from dithiol to polysulfide. Because of the long molecular chain of the products, their values of Rf were lower than that of the initial dithiol monomer. Furthermore, two dots were recorded in the TLC result of dithiol, meaning that being stored in poor conditions, it possibly underwent the process of polymerization.
From the GPC data, the reaction occurred without a doubt, and the molecular weight increased with increasing reaction time, which is consistent with the TLC data (Figure 2). After the 24-hour timescale, the dithiol starting component was entirely reacted, yielding compounds with an M greater than the original.
In regard to the FT-IR spectrum of DI24 after the 24-hour reaction, the characteristic peak of thiol at 2556 cm disappeared. It is tangible that this group was consumed. Moreover, the remaining peaks are similar to those of the initial dithiol monomer corresponding to the attribute of its theoretical structure.
Strikingly, the evaporation of the thiol peaks revealed that complete consumption of this group hardly occurred in the reaction. Given its weak absorption of infrared light, the peaks of the S-H bonds were not demonstrated in the FT-IR results. Another compelling causative factor can be cited to explain this phenomenon. With its high M, there are only two thiol groups in every single molecule located at the end of its chain. It is unsurprising that after the 6-hour reaction, DI06 also witnessed an identical trend. From these analyses, the polysulfide was successfully synthesized via the oxidizing process of thiol with the help of I meaning that our initial goal was achieved.
Obviously, all sizes and shapes of vehicles for gaining polysulfide were illustrated in a deluge of research. For all practical purposes, the emergence of polysulfide can be observed with polyoxyalkylene glycol’s -OH groups and organic acids’ thiol groups undergoing the processes of esterification together with transesterification with the aid of catalysts – saying tetrabutyl titanate 26 at 180-190 °C. Additionally, the reaction of vegetable oil and epichlorohydrin was carried out with the addition of stannic chloride at 160 °C to synthesize polysulfide1. In comparison with the research, our approach is sensationally crude by the dint of being void of reagents coupled with taking place under ambient temperature18. Despite the decided perks, this method shows several shortcomings. It is on account of its high vapor pressure that iodine swiftly sublimates, meaning that it is difficult to employ solid iodine for the experiment at room temperature 27. Using the organic solution in the synthesis is another drawback of the research due to the possibility of adversely affecting the environment28.
CONCLUSIONS
This study successfully synthesized polysulfide with a terminal thiol group. The reaction time was overseen by TLC. After 24 h, the reaction was terminated with a pure polysulfide product. It is information from FT-IR and H-NMR spectra that show that the product was effectively synthesized; moreover, GPC results revealed that the Mn of the synthesized product is approximately 12000 g/mol. This achievement would, more or less, be conducive to the synthesis of self-healing materials
Acknowledgments
This research was fully supported by the MURATA project under grant number 21VH05, and we acknowledgment Ho Chi Minh City University of Technology (HCMUT) and VNU-HCM for supporting this study.
LIST OF ABBREVIATIONS
TLC: thin layer chromatography
FT-IR: Transmission Fourier transform infrared
H-NMR: Proton nuclear magnetic resonance
GPC: Gel permeation chromatography
Mn: Number average molar mass
AUTHOR CONTRIBUTION
Nguyen Khai Hoang Nguyen, Mai Ly Nguyen Thi: methodology, investigation, Duc Anh Nguyen Song, Huan Hoang Dang, Thuy Thu Truong: Investigation, Formal Analysis, Le-Thu T. Nguyen: supervision.

