Solvent free, microwave-assisted synthesis and cytotoxicity evaluation of benzoxazole derivatives
- Department of Chemistry, College of Natural Sciences, Can Tho University, Can Tho, Viet Nam
Abstract
Introduction: This work aims to develop an efficient green synthetic procedure and cytotoxicity evaluation of benzoxazole derivatives, an essential building block in medicinal chemistry research owing to its broad range of pharmacological properties, especially anticancer activity. Methods: The microwave-assisted condensation reaction between 2-amino-4-methylphenol and aromatic aldehydes using iodine as the oxidant under solvent-free conditions was explored. The cytotoxicity of the prepared benzoxazoles against the three human cancer cell lines including Lu-1, Hep-G2 and MCF-7 were examined using a MTT assay. Results: Five 2,5-disubstituted benzoxazole derivatives have been successfully synthesized in good yields (67-90%) using iodine as the oxidant under solvent-free microwave irradiation conditions. The initial cytotoxicity evaluation of the four synthesized compounds revealed that compounds 3b and 3e exhibited good activity against MCF-7 (IC50 = 12 μg/mL) and Hep-G2 (IC50 = 17.9 μg/mL), respectively. Remarkably, compound 3c showed the best cytotoxicity against MCF-7 at IC50 = 4 μg/mL. Conclusion: Microwave irradiation effectively assisted the condensation reaction between 2-amino-4-methylphenol and aromatic aldehydes in the presence of iodine under solvent-free conditions to facilitate the desired benzoxazoles in good total yields. The prepared benzoxazoles proved to be potent candidates for further study in the development of anticancer agents.
INTRODUCTION
Cancer continues to be a leading health problem worldwide and the pursuit of novel clinically useful anticancer agents is therefore one of the top priorities of drug development research 1. Among the nitrogen-based heterocycles, benzoxazole has been known as an essential building block in organic synthesis and has a profound effect on medicinal chemistry research owing to its broad range of pharmacological properties2, especially its anticancer activity3. A number of methods have been reported for the synthesis of benzoxazole derivatives including the condensation of 2-aminophenol or its derivatives with carboxylic acids, nitriles, isocyanates, aliphatic amines, or more commonly, aldehydes2. However, these synthetic routes often require harsh reaction conditions such as the use of strong acids or oxidants and a high reaction temperature. Therefore, the development of an effective route for the synthesis of benzoxazoles is still a noteworthy research focus in the field of synthetic chemistry.
Microwave-assisted reactions under solvent-free conditions are environmentally benign and have attracted much attention in relation to organic synthesis. They possess many advantages over conventional methods such as milder reaction conditions, shorter reaction times, and good yields 4, 5. The utilization of microwave irradiation is beneficial for the synthesis of benzoxazole in recent years6. In view of the facts mentioned above, herein we report an efficient microwave-assisted synthesis and cytotoxicity evaluation of benzoxazole derivatives against the three human cancer cell lines Lu-1, Hep-G2, and MCF-7.
MATERIALS AND METHODS
General information
The reactions were monitored by thin layer according to precoated chromatography (TLC) on 0.2 mm pre-coated silica gel 60 F254 plates (Merck). H-NMR and C-NMR spectra were measured using Bruker Avance 500 MHz and Bruker Avance 600 MHz spectrometers. The chemical shifts are given in parts per million (ppm) relative to tetramethylsilane (MeSi, = 0), and the values are given in Hertz. The HRESI-MS observation was performed on a Sciex OS 1.2 mass spectrometer. FT-IR was conducted using the KBr pellet method on Thermo Nicolet 6700. All chemicals and solvents used in this study were of analytical grade. Reactions under microwave irradiation were performed in a microwave synthesis system (CEM Discovery).
General method for the synthesis of benzoxazole derivatives (3a-f)
A mixture of 2-amino-4-methylphenol 1 (0.5 mmol), aldehydes 2a-f (0.5 mmol), KCO (69 mg, 0.5 mmol), and I (126.9 mg, 0.5 mmol) was subjected to microwave irradiation (CEM Discovery reactor) at 120°C for 10 minutes. A saturated aqueous solution of NaSO(5 mL) was then added to the reaction mixture and the resulting solution was extracted using EtOAc (3×10 mL). The combined organic layers were washed with a saturated solution of NaCl (30 mL), dried over NaSO, and the solvent was evaporated under reduced pressure. Purification of the products was performed using column chromatography.
MTT Proliferation Assay
The cytotoxic activity was performed based on the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) according to the method described by Tim Mosman (1983)7. The cells were kept in liquid nitrogen, activated, and maintained using DMEM (Dulbecco's Modified Eagle Medium) or MEME (Minimum Essential Medium with Eagle salt) containing 7-10% FBS (Fetal Bovine Serum) and other essential ingredients. The cells were grown in a humidified incubator containing 5% COat 37°C in absolute sterility. Cells grown in the liquid phase were used for further toxicity experiments. The compounds were dissolved in DMSO with an initial concentration of 20 mg/mL as a stock. The final concentration was determined to be 128, 32, 8, 2, and 0.5 µg/mL after dilution twice from the initial concentration. Ellipticine was dissolved in DMSO at 0.01 mM and used as a positive control. The cells were dissociated using trypsin, then we added the culture medium at a density of around 1 to 3×10 cells/mL. Briefly, a 10 µL sample and 190 µL cell were added to 96 well-plates followed by incubation at standard conditions. After 72 hrs, 10 µL of MTT (5 mg/mL) was added to each well before further incubation for 4 hrs. The medium was then discarded, and the formazan crystals were dissolved with 100 µL of DMSO. The optical density was read at 540 nm. Cells were grown in a free medium as a positive control and in the culture medium without cells as a negative control. The experiments were repeated at least three times. The concentration (IC values) of the compounds required inhibiting 50% of the growth of the human cancer cell lines which was calculated according to the previously described method 8.
RESULTS
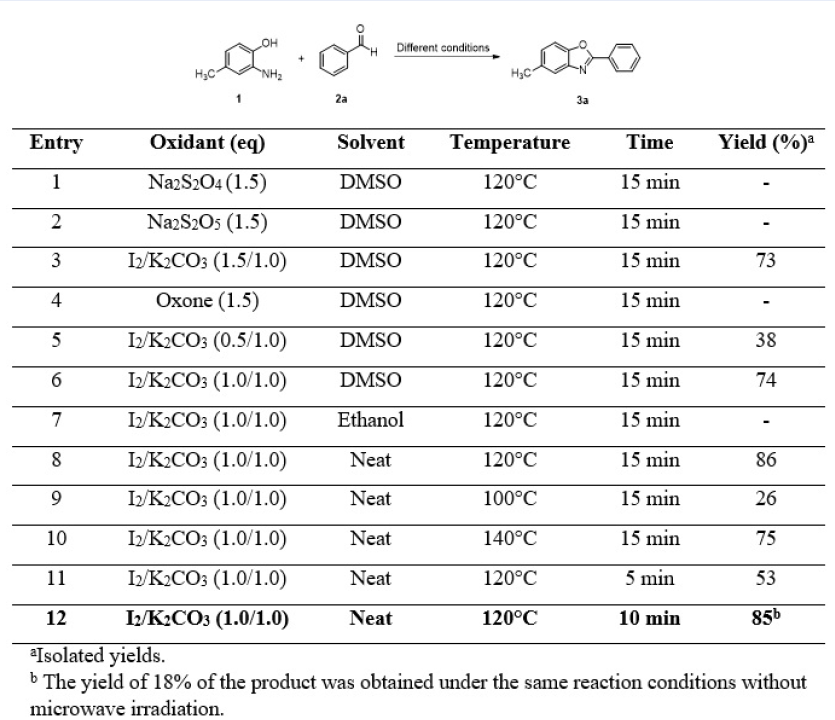
To synthesize the benzoxazoles, we attempted a microwave-assisted condensation reaction between 2-aminophenol or its derivatives and aromatic aldehydes using iodine as the oxidant under solvent-free conditions (Figure 1).

Synthesis of 2,5-disubstituted benzoxazoles
First, the formation of 5-methyl-2-phenylbenzoxazole (3a) was explored using 2-amino-4-methylphenol (1) and benzaldehyde (2a) as the model substrates. The reaction was carried out using the 1:1 molar ratio of (1) and (2a) under different conditions as presented in Figure 2.
The best yield (85%) of the desired product (3a) was obtained by applying the reaction conditions as shown in Entry 12.
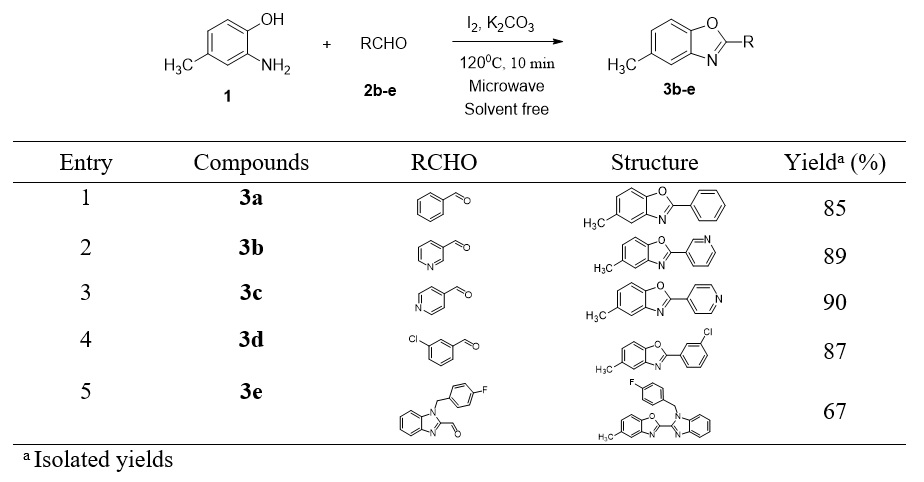
The optimum reaction conditions were then applied for the synthesis of other benzoxazole derivatives without any modification. As shown in Figure 3, the reaction conditions were applicable to different substituted aromatic aldehydes leading to the formation of five corresponding 2,5-disubstituted benzoxazoles (3b-e) with good yields.
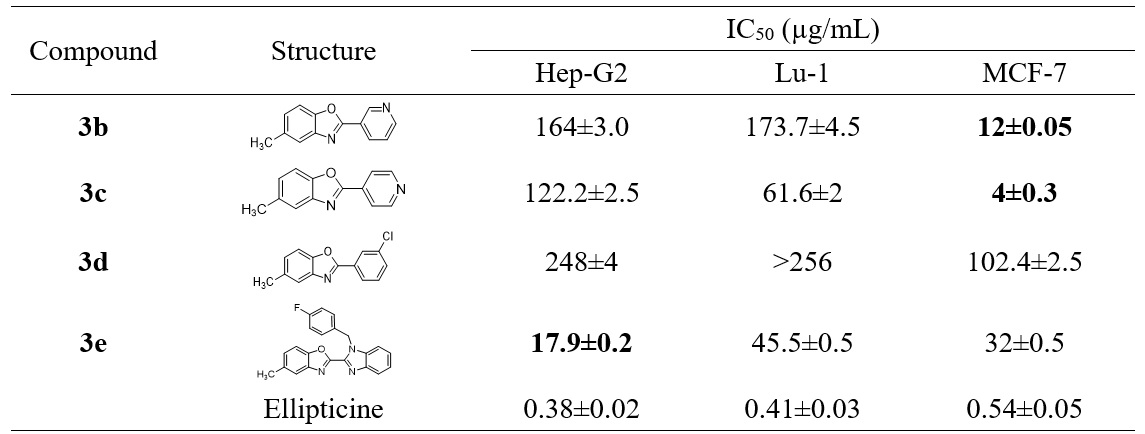
The cytotoxicity of the four prepared benzoxazoles (3b-e) against the three human cancer cell lines including Lu-1 (lung cancer), Hep-G2 (liver cancer), and MCF-7 (breast cancer) were then examined. The results are presented in Figure 4.
DISCUSSION
Iodine was found to be a mild and effective oxidant in the microwave-assisted condensation reactions of 2-amino-4-methylphenol with aromatic aldehydes to result in 2,5‑disubstitued benzoxazole derivatives with good yields. The efficiency of the reaction significantly decreased when replacing I/KCO(1.0/1.0 molar ratio) with other oxidants such as NaSO, NaSO or oxone (Figure 2, Entries 1, 2 and 4, respectively) or when using either DMSO (Entry 6) or ethanol (Entry 7) as the solvent. Additionally, an increase in the amount of iodine did not seem to affect the course of the reaction (Entry 6). Finally, a reaction temperature of 120°C and 10 minutes of irradiation were found to give the best results. Under these conditions, the desired product 5-methyl-2-phenylbezoxazole (3a) was obtained with a good reaction yield of 85%. It is worth noting that under the same reaction conditions without microwave irradiation, the reaction was only completed after 3 hours, providing the same product (3a) at only a 18% yield.
As shown in Figure 3, the optimal reaction conditions were applicable to different substituted aromatic aldehydes. The presence of highly electronegative atoms such as nitrogen (Entry 2 and 3) or chlorine (Entry 4) on the benzene ring resulted in increasing the electrophilicity of the aldehydes in the condensation reaction, thereby leading to an increase in the yields of the desired benzoxazoles 3b-d compared to 3a.
Recently, the combination of two or more pharmacophores of bioactive scaffolds to form hybrid analogues with improved potency has been extensively applied in the development of new drugs or drug candidates. The presence of two or more biologically active pharmacophores in one unit is expected to enhance biological activity as well as increase its ability to interact with more than one biological target9.
Benzimidazole is the isostere of a purine-based nucleic acid. This compound and its derivatives are useful building blocks in the development of bioactive molecules. In particular, benzimidazole is the most prominent heterocycle with cytotoxic properties against different types of cancer cell line 10, 11. Taking this into consideration, we attempted to incorporate the benzimidazole moiety into the benzoxazole system at the C-2 position. To that end, 2-amino-4-methylphenol (1) was then condensed with benzyl benzimidazolecarbaldehyde 2e 12 under the developed optimal reaction conditions to provide the novel benzoxazole/benzimidazole hybrid 3e at a 67% yield. The presence of an electron-withdrawing group (F) on the benzene ring at the -1 position of the benzimidazole moiety seemed to be worse as there was a decrease in the yield of 3e compared to compound 3a observed.
The results for cytotoxicity in Figure 4 indicate that compound 3d bearing the hydrophobic groups (CH, Cl) on the two benzene rings of the benzoxazole structure showed a weak activity against the three tested cancer cell lines. However, the presence of a hydrogen bond acceptor group such as nitrogen on the benzene ring (compounds 3b and 3c) could remarkably change the activity against MCF-7 with the more profound effect being observed with the nitrogen being located at the C-4 position (compound 3c), which showed a IC of 4 µg/mL. The substitution of the phenyl group at the C-2 position of the benzoxazole by a benzimidazole moiety (3e) led to a loss of activity in the MCF-7 cell line but brought about the rather good cytotoxicity against the Hep-G2 cell line (IC of 17.9 µg/mL). These results indicate that benzoxazole derivatives are obviously potent candidates for further study in the development of anticancer agents.
CONCLUSION
Five benzoxazole derivatives have been efficiently synthesized from 2-amino-4-methylphenol with different aromatic aldehydes under microwave irradiation conditions. The use of non-toxic iodine under solvent-free conditions has made this protocol practical, environmentally friendly, and economically attractive. The short reaction time, mild reaction conditions, and high yields of the products are the other advantages of the present method. The initial cytotoxicity evaluation showed that compounds 3b and 3c exhibited good activity against MCF-7 while compound 3e expressed good cytotoxicity against the Hep-G2 cell line. The studies ongoing in our lab include checking the effects of other pharmacophoric groups at different positions in the benzoxazole system for cytotoxicity as well as the mechanism of the activity being reported in due course.

Optimization of the reaction conditions
Microwave-assisted synthesis of benzoxazoles under solvent-free conditions
Cytotoxicity of the prepared benzoxazoles
LIST OF ABBREVIATION
Lu-1: human lung cancer cell line
Hep-G2: human liver cancer cell line
MCF-7: human breast cancer cell line
IC: concentration that causes 50% inhibition of cancer cell growth.
COMPETING INTERESTS
The authors declare that they have no competing interests.
Author Contributions
Bui Thi Buu Hue conceived the idea and designed the works. Tran Quang De and To Thi Diem Sương performed chemical synthesis, and structure identification. All authors read and approved the final manuscript for publication.
SPECTRAL DATA OF BENZOXAZOLES (3a-f)
5-Methyl-2-phenylbenzo[]oxazole (3a): Yield 85% as white solid. R= 0.52 (Hex:EtOAc = 9:1). Mp. 104-106°C. FT-IR (KBr) ν (cm): 3054, 2920, 2854, 1552, 1476, 1446, 1335, 1285, 1265, 1199, 1055, 1019, 927, 824, 797, 701, 686. HR-ESI-MS: 210.0842 [M+H] (calcd for CHNO 210.0919). H-NMR (500 MHz, DMSO-) : 8.24-8.26 (m, 2H, >CH), 7.57 (s, 1H, >CH), 7.51-7.54 (m, 3H, >CH), 7.45 (d, 8.5 Hz, 1H, >CH), 7.17 ( 8.5 Hz= 1.5, 1H, >CH), 2.49 (s, 3H, -CH). C-NMR (125 MHz, DMSO-) : 163.1, 149.0, 142.3, 134.4, 131.4, 128.9, 127.5, 127.3, 126.3, 119.9, 109.9, 21.5.
5-Methyl-2-(pyridin-3-yl)benzo[]oxazole (3b): Yield 89% as pink solid. R= 0.22 (Hex:EtOAc = 4:1). Mp. 103-105°C. FT-IR (KBr) ν (cm): 3055, 3017, 2922, 1676, 1598, 1677, 1552, 1480, 1412, 183, 1341, 1268, 1197, 1073, 1020, 928, 810, 716, 707. HR-ESI-MS: 211.0799 [M+H] (calcd for CHNO: 211.0871H-NMR (500 MHz, DMSO: 9.33 (1.5 Hz, 1H, >CH), 8.79 ( Hz Hz 1H, >CH), 8.51 ( 8.0 Hz 2.0 Hz 1H, >CH), 7.69 ( 8.5 Hz, 1H, >CH), 7.63-7.66 (, 2H, >CH), 7.28 ( 8.5 Hz 1.0 Hz 1H, >CH), 2.45 (, 3H, -CH).C-NMR (125 MHz, DMSO 160.4, 152.2, 148.5, 147.9, 141.4, 134.6, 134.5, 126.9, 124.3, 122.9, 119.7, 110.5, 20.94.
5-Methyl-2-(pyridin-4-yl)benzo[]oxazole (3c): Yield 90% organe yellow solid. R= 0.22 (Hex:EtOAc = 4:1). Mp. 136-138°C.FT-IR (KBr) (cm): 3037, 2922, 2856, 1616, 1540, 1473, 1412, 1342, 1296, 1071, 1062, 988, 930, 832, 811, 701, 695, 513.HR-ESI-MS: 211.0795 [M+H] (calcd for CHNO: 211.0871). H-NMR (500 MHz, DMSO: 8.83 (4.5 Hz 1.5 Hz, 2H, >CH), 8.08 (4.0 Hz 1.5 Hz 2H, >CH), 7.72 (8.5 Hz 1H, >CH), 7.67 ( 1.0 Hz, 1H, >CH), 7.32 (8.5 Hz1.0 Hz, 1H, >CH), 2.46 (, 3H, -CH). C-NMR (125 MHz, DMSO 160.3, 150.8, 148.6, 141.3, 134.8, 133.5, 127.6, 120.7, 120.1, 110.7, 20.9.
2-(3-Chlorophenyl)-5-methylbenzo[]oxazole (3d): Yield 87% as light pink solid. R= 0.53 (Hex:EtOAc = 9:1). Mp. 101-103°C.FT-IR (KBr) (cm): 3067, 3022, 2921, 2860, 1552, 1475, 1434, 1407, 1254, 1192, 1102, 1056, 941, 836, 812, 792, 749, 721, 677. HR-ESI-MS: 244.0528 [M+H] (calcd for CHClNO: 244.0529). H-NMR (500 MHz, DMSO: 8.11-8.13 (m, 2H, >CH), 7.61-7.70 ( 4H, >CH), 7.26 ( Hz Hz 1H, >CH), 2.44 (, 3H, -CH). C-NMR (125 MHz, DMSO 160.8, 148.5, 141.5, 134.5, 133.9, 131.5, 131.3, 128.5, 126.9, 126.6, 125.7, 119.7, 110.4, 20.9.
2-(1-(4-Fluorobenzyl)-1-benzo[]imidazol-2-yl)-5-methylbenzo[]oxazole (3e): Yield 67% as light yellow solid. R= 0.34 (Hex:EtOAc = 4:1). Mp. 175-177°C. FT-IR (KBr) (cm): 3054, 2922, 2852, 1696, 1603, 1509, 1439, 1331, 1268, 1216, 1161, 1092, 1013, 848, 811, 739, 580. HR-ESI-MS: 358.1338 [M+H] (calcd for CHFNO: 358.1356). H-NMR (500 MHz, DMSO: 7.95-7.96 (, 1H, >CH), 7.57-7.60 ( 2H, >CH), 7.36-7.41 ( 3H, >CH), 7.27-7.28 (, 3H, >CH), 6.95-6.98 (, 2H, -OCH), 6.21 (, 2H, -CH), 2.50 (, 3H, -CH). C-NMR (125 MHz, DMSO 163.3, 161.4, 154.3, 148.6, 141.5, 139.7, 136.0, 135.3, 132.0; 128.9, 128.8, 128.1, 125.4, 124.0, 121.3, 120.6, 115.9, 115.7 110.9, 110.8, 48.2, 21.5.

