Effect of Applied Voltage on the Electrochemical Copolymerization of Thiophene and Dithenopyrrole Derivatives
- National Key Laboratory of Polymer and Composite Materials, Ho Chi Minh City University of Technology, Vietnam National University (VNU-HCM), 268 Ly Thuong Kiet, District 10, 70000 Ho Chi Minh City, Vietnam
- National Key Laboratory of Polymer and Composite Materials, Ho Chi Minh City University of Technology, Vietnam National University (VNU-HCM), 268 Ly Thuong Kiet, District 10, 70000 Ho Chi Minh City, Viet Nam
- Faculty of Materials Technology, Ho Chi Minh City University of Technology, Vietnam National University, 268 Ly Thuong Kiet, District 10, 70000 Ho Chi Minh City, Vietnam
- Faculty of Materials Technology, Ho Chi Minh City University of Technology, Vietnam National University, 268 Ly Thuong Kiet, District 10, 70000 Ho Chi Minh City, Viet Nam
- Faculty of Chemical Engineering, Ho Chi Minh City University of Technology, Vietnam National University, 268 Ly Thuong Kiet, District 10, 70000 Ho Chi Minh City, Viet Nam
Abstract
The linear sweep voltammetry potentiostatic approach was employed to electrochemically synthesize the conjugated copolymers P(3HT-random-HPDTP) at three distinct applied voltages (1.4, 1.435, 1.6 V). In relation to their synthesis conditions, their chemical structural, optical, and electrical properties were investigated and compared using Fourier transform infrared (FTIR) spectroscopy, UV‒Vis absorption spectroscopy, and cyclic voltammetry (CV). The results indicated that choosing a suitable applied voltage in the electropolymerization process significantly improved the electrochemical stability and lowered the HOMO level of the conjugated copolymer.
INTRODUCTION
The field of conductive polymers has recently attracted much attention from many researchers due to their potential in various applications, such as anti-static and anti-corrosion coating materials, batteries and supercapacitors, solar cells, sensors, light emitting diodes, and transparent electrode materials.1, 2, 3 Thiophene and dithienopyrrole are among the promising building blocks for the development of high-performance conjugated polymers for organic electronic devices because of their excellent planarity and strong electron-donating properties.4, 5, 6 Additionally, their fused-ring structure minimizes chain distortion, resulting in planarization of the polymer chain and improved 𝜋–𝜋 stacking. Furthermore, P3HT has been used as a model system for fundamental study due to its distinctive hair-rod structure, which comprises a hard conjugated backbone and flexible alkyl side chains that are effective for solubilization.7, 8
In recent years, the development of high-performance conjugated polymers by copolymerization has also shown promise. Copolymerization is widely used to develop novel conjugated polymers with physical and chemical properties that are distinct from and in between those of precursor homopolymers. However, a copolymer significantly differs from a composite and a blend. Jin . performed electropolymerization (EP) of styrene and pyrrole monomers and found that the product polymer was a copolymer of pyrrole and styrene instead of a composite or blend of polypyrrole and polystyrene. Copolymerization has recently been demonstrated to be capable of modifying the wavelength range of light absorption, frontier energy levels, and solubility of conjugated polymers.9, 10 Recently, Acker designed the novel N-anisylphenothiazine–bithiophene conjugated copolymer p(APT-T2), which could be a promising material to design an electrode for energy storage applications due to its intrinsic conductivity combined with the presence of defined redox centers.11 In 2020, Lin . designed six conjugated copolymers using thiophene as a fundamental unit, which all exhibited significantly improved electrochemical stability and noteworthy color changes when transitioning from the oxidized to neutral state with increasing conjugation length.12 In the same year, Zhen Xu and coworkers synthesized a novel copolymer PEM-11 via electrochemical copolymerization of two donor-acceptor type monomers, which exhibited stable n-doping, favorable cycle stability, and a lower electrochemical/optical band gap. The results suggested that the obtained copolymer could be a promising candidate for electrochromic windows, e-papers, or eyewear applications.13 Furthermore, in 2022, Hao Dong . reported a series of D-A copolymers with naphthodithiophene (NDT) as the electron donor (D) and benzodipyrrolidone (BDPP) as the electron acceptor (A). The four D-A copolymers showed excellent thermal stability, and the thermal decomposition temperature was above 380 °C.14
Moreover, another well-known technique for creating polymer films using an electric field is electrochemical polymerization, which is straightforward, fast, efficient, and cost-effective. In contrast to the conventional method, which requires harsh conditions (high temperature and inert environment) and can take several hours or days, the electropolymerization approach is highly effective for synthesizing polymer films because the reaction can be carried out under ambient conditions and in a matter of seconds or minutes. Additionally, the prepared films only formed on the electrodes without any obvious signs of a reaction in the solution. Moreover, the electropolymerization approach uses inexpensive supporting electrolytes instead of specialized catalysts and expensive complexants for solution-phase synthesis12, 15, 16. However, the choice of applied voltage in the electropolymerization process can significantly affect the growth rate and quality of the produced polymer. Higher applied voltages can lead to faster growth rates but lower quality films, while lower applied voltages can result in higher quality films but slower growth rates. Therefore, the applied voltage should be carefully considered balancing the growth rate and quality of the polymers.
In comparison to polydithienopyrrole, polythiophene has a better redox potential and is more affordable, making it a potentially useful material for optoelectronic applications.17 Nevertheless, due to their decreased backbone planarity and disordered molecule packing with irregularity restricting their application, the regio-random chemical structure of polythiophene and its derivatives has been found to only provide limited charge transfer in the solid state.18 Dithiopyrrole, on the other hand, has long been recognized for its distinct features, including exceptional thermal and electrical stability due to its broad HOMO-LUMO energy gap. Consequently, we attempted to use electrochemical copolymerization at various applied voltages to incorporate thiophene with dithienopyrrole units. Herein, thiophene and dithienopyrrole derivatives were used to prepare conjugated copolymers at three distinct applied voltages of 1.4, 1.435, and 1.6 V via the linear sweep voltammetry potentiostatic technique. The relationship between the electropolymerization conditions and the electrochemical properties of the conjugated copolymers was then studied in detail.
EXPERIMENTAL
Materials
3-hexylthiophene (99%), 4-(4-hexylphenyl)-4H-dithieno[3,2-B:2’,3’d]pyrrole (98%), and lithium perchlorate (LiClO, 98%) were purchased from Acros Organic and used as received.
Chloroform (CHCl, 99.5%) was purchased from Fisher/Acros and dried using molecular sieves under N. Acetonitrile (CHCN, 99%) and methanol (99%) were purchased from Fisher/Acros and used as received.
Characterization
FTIR spectra, collected as the average of 64 scans with a resolution of 4 cm, were recorded from the KBr disk on the FTIR Bruker Tensor 27. UV–vis absorption spectra of copolymers in solution were recorded on an ocean optic USB2000 spectrometer over a wavelength range of 175 nm – 800 nm. AUTOLAB equipment (Utrecht, Netherlands) was used to measure the electrochemical properties of the molecules. The AUTOLAB software is NOVA 1.11. The electrochemical experiments used ITO glass as a working electrode, Pt as a counter electrode and a Ag/Ag reference electrode. The solutions were prepared with 0.1 M TBAPF (tetrabutylammonium hexafluorophosphate) in acetonitrile. Ag/Ag reference electrode (0.1 M AgNO/0.1 M TBAPF in CHCN; 0.320 V and SCE) was used as a reference potential.
Characterize the electrochemical properties of monomers 3-hexylthiophene (3HT) and4-(4-hexylphenyl)-4H-dithieno[3,2-B:2’,3’d] pyrrole (HPDTP)
First, the electrochemical experiment of monomer 3-hexylthiophene (3HT) was investigated by adding 3HT (0.27 mL; 1.5 mmol) and LiClO (319.2 mg; 3.0 mmol) to acetonitrile (30 mL) in an electrochemical cell, employing an indium tin oxide (ITO) glass as a working electrode and platinum disk as a counter electrode. Then, the mixture was subjected to cyclic voltammetry at a voltage of (-2) – 2 V and a scan rate of 0.1 mV/s.
The same experiment was applied for monomer 4-(4-hexylphenyl)-4H-dithieno[3,2-B:2’,3’d] pyrrole (HPDTP) to the electrochemical cell HPDTP (20.4 mg; 0.06 mmol), LiClO (319.2 mg; 3 mmol) and acetonitrile (30 mL). The electrochemical experiment was conducted using ITO glass and Pt as the working and counter electrodes, respectively, with an applied voltage of (-2) – 2 V and a scan rate of 0.1 mV/s.
Electrochemical copolymerization of 3HT and HPDTP at different applied voltages
For electrochemical polymerization of the copolymers from 3HT and HPDTP, the electrolyte consisted of monomer 3HT (0.27 mL; 1.5 mmol), monomer HPDTP (20.4 mg; 0.06 mmol), and LiClO (319.2 mg; 3 mmol) dispersed in acetonitrile (30 mL). ITO glass and Pt were used as the working and counter electrodes, respectively. The copolymers P1, P2, and P3 were electrochemically polymerized at three different voltages of 1.4, 1.435, and 1.6 V, respectively, for approximately 5 minutes. After that, the thin films of the three copolymers on ITO glass were soaked in acetonitrile for 30 minutes to remove small molecules and dried in a vacuum oven at 50 °C for 24 hours.
RESULTS AND DISCUSSION
Electrochemical copolymerization of 3HT and HPDTP
Before conducting the electrochemical copolymerization to synthesize copolymers P1, P2, and P3, the onset oxidation potentials (E) of monomers 3HT and HPDTP were examined from their cyclic voltammograms (Figure 1).

Cyclic voltammograms (CV) of monomers 3HT and HPDTP with the range of voltage from (-2) – 2 V and a scan rate of 0.1 mV/s.
The acquired current-time characteristic curves for the electrochemical copolymerizations to synthesize copolymers P1, P2, and P3 are shown in Figure 2.

Current-time graphs of the electrochemical copolymerizations to synthesize copolymers P1 (top, left), P2 (top, right), and P3 (bottom) at voltages of 1.4, 1.435, and 1.6 V, respectively.
After the electropolymerizations were performed successfully, the chemical structures of copolymers P1, P2, and P3 were characterized using FTIR spectroscopy, and the results are shown in Figure 3.

Comparison of the FTIR spectra of copolymers P1, P2, and P3.
Optical properties of the copolymer P(3HT-random-HPDTP)
Next, the optical properties of the product copolymer were characterized using UV‒Vis absorption spectroscopy. The resulting chemical structure indicated that copolymers P1, P2, and P3 are the same compound. Therefore, copolymer P1 was used as a sample to analyze the optical properties of the prepared copolymer. The UV‒Vis absorption spectra of the copolymer and P(HPDTP) are shown and compared in Figure 4 to investigate the effect of the expanded - conjugated system on the optical properties of the synthesized copolymer.

UV–Vis absorption spectra of copolymer
Electrochemical properties of copolymer P(3HT-random-HPDTP)
By employing the cyclic voltammetry (CV) technique, the electrochemical behaviors of copolymers P1, P2, and P3 were investigated to better understand their electrochemical activity.
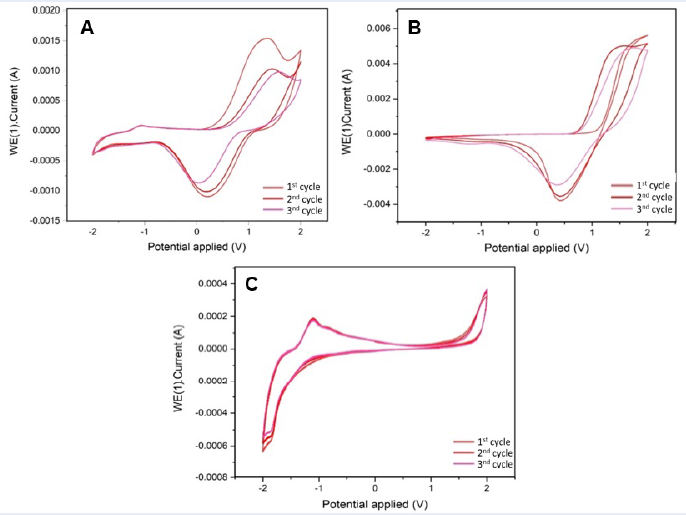
The results of the electrochemical behaviors of P1, P2 and P3 are presented in Figure 5.

Cyclic voltammograms of copolymers P1 (A), P2 (B), and P3 (C) cycled 3 times at a potential scan rate of 5 mV/s.
DISCUSSION
Figure 1 demonstrates the E of the 3HT and HPDTP, which began at 1.4 and 0.8 V, respectively, and HPDTP also had oxidation peaks at 1.0 and 1.6 V. As a result, the applied voltage for electropolymerization should be above the E and under the overoxidation of both monomers, which ranges from 1.4 to 1.6 V. Consequently, copolymers P1, P2, and P3 were prepared by a linear sweep voltammetry potentiostatic technique with an applied voltage of 1.4 V (start point), 1.435 V (intersection point), and 1.6 V (endpoint).
As seen in Figure 2, the current intensities decrease gradually with increasing reaction time for all three electropolymerizations. The prepared conductive copolymers exhibited good electrochemical redox activity, as evidenced by the significant growth of the corresponding conductive copolymers on the ITO glass.
FTIR spectroscopy was scanned in the frequency region of 600-4000 cm at room temperature using an infrared spectrometer by employing the KBr pellet technique. The copolymers P1, P2, and P3 all have distinctive bands that are comparable, as seen in Figure 3 and
IR absorption wavenumbers (cm-1) and their functional groups of copolymers P1, P2, and P3
|
P1 |
P2 |
P3 |
Functional group |
|
Wavenumbers (cm-1) |
| ||
|
784 |
748 |
785 |
Aromatic C-H bend |
|
1006 |
1031 |
1006 |
C-S stretch |
|
1257 |
1332 |
1257 |
C-N Aromatic strech |
|
1512 |
1514 |
1458 |
Methyl and methylene C-H bend |
|
2960 |
2923 |
2960 |
Methyl and methylene C-H stretch |
The optical properties of the copolymer are analyzed and shown in Figure 4. According to Figure 4, the copolymer exhibits broad wavelength absorption from 300 nm to 550 nm, which is longer than P(HPDTP), 350 nm to 550 nm. The longer wavelength absorption is due to the — excitation of the expanded -conjugation of the copolymer. Moreover, as the size increases, the copolymer intensity absorption is much larger than that of polymer P(HPDTP).
The energy bandgap, which can be identified using the UV‒Vis absorption spectrum, is another significant parameter for evaluating the optical characteristics of the conjugated copolymer. Using the Plank equation (Eg(opt) = 1240/λ), the calculated energy bandgap of the copolymer is 2.33 eV, which is larger than the value of P(HPDTP), 2.02 eV, because of the increased number of conjugated bonds in the copolymer’s structure. The outcome shows that the copolymer has a high optical bandgap value, which makes it advantageous for high-temperature optical devices such as LEDs, lasers, or solar cells.
According to Figure 5, copolymers P1 and P2 exhibited distinct redox peaks with hysteresis (potential drift) between the anodic and cathodic peak potentials, which may be ascribed to several factors, including local polymer chain rearrangement, slow mutual transformation of various electronic species, slow heterogeneous electron transfer, and electronic charging of the interfacial exchange at the polymer/solution and metal/polymer interfaces. These factors can cause slow and complex electrochemical reactions, which can lead to hysteresis between the anodic and cathodic peak potentials19. However, the redox activity of copolymer P3 demonstrated irreversible behavior at a scan rate of 5 mV/s, which was attributed to the molecular size of the copolymer. With a larger molecule size, copolymer P3 exhibited a lower diffusion rate than copolymers P1 and P2.20
Additionally, an empirical equation was used in conjunction with cyclic voltammetry to evaluate the HOMO and LUMO energy levels of the copolymers, and the results are shown in
Electrochemical properties and calculation parameters of copolymers P1, P2, and P3
|
Copolymer |
|
HOMO* (eV) |
LUMO* (eV) |
Eg(opt) (eV) |
|
P1 |
0.49 |
-4.89 |
-2.87 |
2.02 |
|
P2 |
1.17 |
-5.57 |
-3.55 |
2.02 |
|
P3 |
1.63 |
-6.03 |
-4.01 |
2.02 |
|
*HOMO = -(4.4 + | ||||
As illustrated in Figure 5, the stability of the three copolymers was also examined, with a total of three cycles performed. The redox activities of copolymers P1 and P2 were sustained at approximately 60% and 80%, respectively, after three cycles of scanning, indicating generally excellent redox activity. On the other hand, after three scanning cycles, the electrochemical stability of copolymer P3 reaches a stability of over 97%. This improved electrochemical stability was achieved by increasing the chain length of the copolymer, which benefits by raising the applied voltage of the electropolymerization of copolymer P3.
CONCLUSION
3HT-HPDTP random conjugated copolymers were effectively constructed at different applied voltages of 1.4, 1.435, and 1.6 V by electrochemical copolymerization using a linear sweep voltammetry potentiostatic technique. The structural, optical, and electrochemical properties of the prepared copolymers were then examined and compared. With an increase in the applied voltage during electropolymerization, the HOMO levels of copolymers P1, P2, and P3 gradually decrease. Moreover, the electrochemical stability of copolymer P3 was significantly improved, reaching above 97% when prepared at an applied voltage of 1.6 V. The preparation of high electrochemical stability copolymers for a variety of optoelectric applications using the linear sweep voltammetry potentiostatic technique has been demonstrated to be highly adaptable, straightforward, and effective.
ACKNOWLEDGMENT
This research was fully supported by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number ‘‘104.02-2020.39”.
Abbreviations
Proton nuclear magnetic resonance (1H NMR), Fourier transform infrared (FT-IR) spectroscopies, gel permeation chromatography (GPC), deuterated chloroform (CDCl), 4-(4-hexylphenyl)-4H-dithieno[3,2-B:2’,3’d] pyrrole (HPDTP), cyclic voltammetry (CV).
Acknowledgments
This research was fully supported by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number ‘‘104.02-2020.39”.
Author’s contributions
Thao Phuong Le Nguyen, Cam H. T. Nguyen and Ha Tran Nguyen conceptual the project methodology, wrote the original draft and supervised the investigation. Thao Thanh Bui, Bao Kim Doan, Tam Hoang Luu, Chau Duc Tran, Thuy Thu Truong, Tung Viet Tuan Tran, Hai Le Tran, Xuan Van Mai analysed experimental data. H. All authors discussed and edited the manuscript.
Competing interests
The authors declare that they have no competing interest.

