Highly pure alpha-hederin from Hedera helix leaves isolated by centrifugal partition chromatography
- Institute of Applied Materials Science, Vietnam Academy of Science and Technology (VAST), Ho Chi Minh City, 70000, Vietnam
- Hoang Hoa Tham High School, Ho Chi Minh City, 70000, Vietnam
- Center for Research and Technology Transfer, Vietnam Academy of Science and Technology (VAST), Ha Noi City, 100000, Vietnam
- National Institute of Medicinal Materials, Ha Noi City, 100000, Vietnam
Abstract
Introduction: Hedera helix is a species of the Araliaceae family, which contains flavonoids, coumarins, phenolic acids, sterols, alkaloids, anthocyanins, and triterpene saponins. α-Hederin is a saponin compound of the H. helix that possesses many potential biological activities. In previous studies, several methods were used to isolate and purify α-hederin from ivy leaves. However, the loss of α-hederin obtained during solid chromatography purification was relatively high, and it was difficult to obtain α-hederin with high purity. In this research, a centrifugal partition chromatography (CPC) method was established for the isolation of α-hederin directly from ethanol crude extract of Hedera helix leaves from Da Lat, Lam Dong province, Vietnam.
Methods: The separation of α-hederin was carried out by applying centrifugal partition chromatography with a PLC 2250 system (Gilson Inc., France) coupled with an SCPC-250 column (Armen Instrument, France). In a CPC run, a two-phase solvent system consisting of n-hexane–ethyl acetate–methanol–water (2:3:2:3, v/v) in ascending mode (the lower phase was the stationary phase), with a rotation speed of 1600 rpm and a flow rate of 8.0 mL/min, was used.
Results: The purity of the isolated α-hederin was 95.7%, as confirmed by HPLC-DAD at 210 nm. The structure of purified α-hederin was determined by 1H-NMR and 13C-NMR. The yield of α-hederin was 12.7%.
Conclusion: This investigation might be used as a reference for the large-scale separation of high-purity α-hederin from H. helix leaves.
INTRODUCTION
Ivy ( L. ), one of 15 species in the genus , is an evergreen dioecious woody liana that belongs to the Araliaceae family1. The chemical composition of leaves contains flavonoids, coumarins, phenolic acids, sterols, alkaloids, anthocyanins, and triterpene saponins1, 2, 3, 4. Saponins from ivy leaves exhibit anthelmintic, antifungal, antimutagenic, antileishmanial, antioxidant, and anti-inflammatory activities5, 6, 7, 8, 9, 10. α-Hederin (Figure 1) is one of the effective saponins of leaves and has numerous bioactivities, including antileishmanial8, anti-inflammatory, antifungal, anticancer, antispasmodic, and hepatoprotective activities10, 11, 12, 13, 14.
Centrifugal partition chromatography (CPC) is a liquid‒liquid partition chromatographic technique that has the advantages of a faster separation time, no sample adsorption, and the ability to purify a wide range of phytochemicals using a variety of solvent systems. Because of these merits, CPC is widely used in the preparative separation of natural products15. The liquid stationary phase is immobilized in the CPC column, and the sample components are partitioned between the two phases and separated using partition coefficient differences. Only a restricted number of solvents are employed in CPC. The most common solvents are n-hexane, ethyl acetate, methanol, and water16.
The present study aimed to establish an efficient CPC method for the purification of α-hederin from leaf extract.
MATERIALS AND METHODS
Chemicals and reagents
n-hexane, ethyl acetate (EtOAC), chloroform (CHCl), methanol (MeOH), acetonitrile (ACN) of HPLC grade, formic acid of analytical grade, dimethyl sulfoxide (DMSO) (Scharlau, Spain); deionized water for HPLC; sulfuric acid for analysis (Merck, Germany); ethanol absolute (Chemsol, Vietnam); α-hederin (Natpro, Vietnam).
Methods
Extraction of plant materials
leaves were collected from Da Lat, Lam Dong province, Vietnam. Approximately 1.0 kg of leaves were cut into small pieces and extracted in 5.0 l of ethanol for 24 hours at room temperature. After vacuum evaporation of methanol using a rotavapor at 60°C, the crude extract was obtained and later used for CPC separation of α-hederin.
Thin layer chromatography (TLC)
TLC was performed on silica gel plates (Si60 F254, 100 x 200 mm, 0.2 mm layer thickness, Merck, Germany). The crude extract of ivy leaves and standard α-hederin were monitored on TLC plates. CHCl–MeOH (4:1, v/v) was used as the mobile phase. After developing, the plate was sprayed with TLC reagent (HSO in 10% ethanol) and heated at 105°C for a minute. Then, light purple α-hederin spots appeared.
Selection of a two-phase solvent system
The two-phase solvent system was chosen according to the partition coefficient (K) value of α-hederin. In this experiment, approximately 10 mg of the crude extract was dissolved in 4 mL of the preequilibrated two-phase solvent system consisting of n-hexane–ethyl acetate–methanol–water with three different ratios of Arizona systems: J (2:5:2:5), L (2:3:2:3), and N (1:1:1:1). Both the upper and lower phases were analyzed by TLC to determine the partition coefficient values of α-hederin. For efficient CPC separation, the appropriate K value was between 0.2 and 5.0 and was preferably 1.017, 18.
CPC separation method
CPC separation of the ivy extract was performed with a system comprising an SCPC-250 column (Armen Instrument, France) coupled with a PLC 2250 system (Gilson Inc., France) built in with a quaternary pump, a diode array detector, and a fraction collector. The total column volume was 250 mL. Gilson Glider CPC Software V5.1d.01 (Gilson Inc., France) was used to control the instrumentation.
In this experiment, the CPC method was established with an optimized Arizona L biphasic solvent system in ascending mode. First, the CPC column was filled with the lower stationary phase (n-hexane-EtOAc-MeOH-HO, 0:16:32:52, v/v) at a flow rate of 30.0 mL/min with a rotation speed of 800 rpm. Then, the upper mobile phase (n-hexane-EtOAc-MeOH-HO, 45:48:6:1, v/v) was pumped into the column at a flow rate of 8.0 mL/min with a rotation speed of 1600 rpm. Equilibrium was reached when the mobile phase emerged from the CPC system.
Approximately 100 mg of extract was dissolved in 2 mL of the Arizona L system. The whole sample mixture was injected through a 2.0 mL loop for α-hederin separation. UV detection was monitored at 210 nm. The fractions were collected in 32 mL tubes (5 mL for each fraction) in the collector.
HPLC analysis and quantification of α-hederin
crude extract and CPC fraction peaks were analyzed by HPLC-DAD using the method described in our previous study19. The Hewlett Packard 1050 HPLC system (Agilent Technologies, USA) with an Inertsil ODS-3 column (250 x 4.6 mm; 5 µm) (GL Sciences, Japan) was used at a flow rate of 1.0 mL/min, with a gradient solvent system of water containing 0.1% formic acid (A) and acetonitrile (B). The mobile phase gradient was performed as follows: 0-20 min: from 80% to 40% A inB; 20-24 min: from 40% to 0% A in B; 24-30 min: 100% B. The sample injection volume was 20.0 µL. The effluents were monitored at 210 nm. Data were analyzed using ChemStation for LC 3D software (Agilent Technologies, USA).
NMR analysis
NMR experiments of the isolated compound were carried out using a Bruker Avance III spectrometer with DMSO as the solvent and TMS as the internal standard.
RESULTS
Selection of a two-phase solvent system
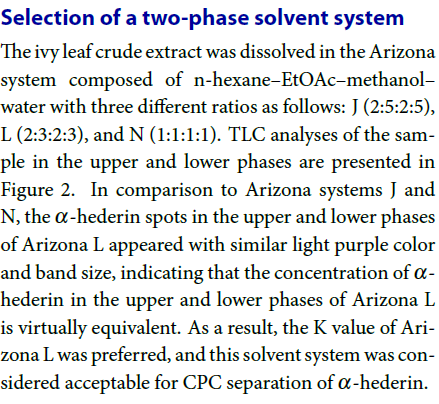
The ivy leaf crude extract was dissolved in the Arizona system composed of n-hexane–EtOAc–methanol–water with three different ratios as follows: J (2:5:2:5), L (2:3:2:3), and N (1:1:1:1). TLC analyses of the sample in the upper and lower phases are presented in Figure 2. In comparison to Arizona systems J and N, the α-hederin spots in the upper and lower phases of Arizona L appeared with similar light purple color and band size, indicating that the concentration of α-hederin in the upper and lower phases of Arizona L is virtually equivalent. As a result, the K value of Arizona L was preferred, and this solvent system was considered acceptable for CPC separation of α-hederin.
CPC separation and HPLC analysis
After performing HPLC-DAD analysis, the crude extract containing α-hederin (RT = 21.88) was subjected to CPC separation. Figure 3 shows the HPLC-DAD analysis result of the ivy crude extract at a UV wavelength of 210 nm.
The crude extract of was separated by a single CPC run using a solvent system composed of n-hexane–EtOAc–methanol–water (2:3:2:3, v/v). Identification of the CPC peak fractions was performed by HPLC-DAD analysis. After a one-step operation of CPC, a high purity of α-hederin was achieved at 95.7%. The isolated compound was a white powder, TLC Rf: 0.5. The eluted solvent system was CHCl–MeOH (4:1, v/v). The chemical structure of the isolated compound was confirmed by NMR (Table 1).
Figure 4 shows the HPLC-DAD analysis of the extract and the isolated α-hederin (Rt: 21.88 min). The yield reached 12.7% compared to the α-hederin content in the total extract.
DISCUSSION
NMR analysis of the isolated compound
The H-NMR spectrum (DMSO-d6, 500 MHz) of the compound had three signals of the CH group at δ 0.57(s); 1.07(s); 1.08(s), three CH groups give 1 at δ 0.87(s), 1 oxymethylene H-23 group at δ 3.12 and 3.28, and 1 oxymethin H-3 group at δ 3.49 (1H, m). The H-NMR spectrum also showed the presence of an olefin proton signal at δ 5.16, which is specific for H-12 in the structure of the 5-ring triterpenes.
The C-NMR and DEPT spectra of the compound showed resonance signals of 41 carbons, 30 of which are consistent with published data for the structure of hederagenin consisting of 7 methyl group signals, 11 methylene group signals, 5 methine group signals, and 8 quaternary carbon signals, including the signal of C-12 olefin carbon at δ 121.58 for methine carbon and C-13 quaternary carbon at δ 143.86, also confirming the existence of a double bond in the ring at the C-12 position and a C-28 signal at δ 178.62 of the carboxyl group. In addition, the remaining 11 carbon signals showed the presence of 2 sugar units with resonance signals of 2 anomeric carbons C-1ʹ at δ 102.97 and C-1ʹʹ at δ 99.94, 7 methine group signals, 1 C-5ʹ methylene group signal, and 1 C-6ʹʹ methyl group signal at δ 17.77.
H-NMR and HSQC spectra also confirmed that two anomeric protons interacted with the above 2 anomeric carbons at δ 5.06 (1H, s) and δ 4.63 (1H, d, 6.0). On the H-NMR spectrum, a doublet signal of the methyl group at δ 0.71 showed the existence of a deoxy sugar in the structure of the compound.
The protons in the ring of the sugar units were correctly assigned thanks to the COSY, HSQC, and HMBC spectra starting from the identified anomeric protons. The chemical shift of C-3 (δ 79.32) in the C-NMR spectrum indicated that the sugar chains were attached to this site. Connecting to the sugar units was confirmed by HMBC interactions between the anomeric proton (H-1') of arabinose (δ 4.63) with C-3 (δ 79.32), H-1" of rhamnose (δ 5.06), and C-2' of arabinose (δ 74.28). By comparison to the reference20, the isolated compound was determined to be α-hederin.
Advantages of CPC in α-hederin isolation and purification
Saponins are phytochemicals mostly found in plants and are also present in most vegetables, beans, and herbs. It is a serious challenge in saponin extraction and separation because of their unique structural characteristics. In addition, it is difficult to isolate saponins since there are a mixture of saponin compounds present in plants with similar structures and polarities. Some saponins may undergo enzymatic hydrolysis during water extraction, while esterification of acidic saponins may also occur at some stage in alcohol extraction thus, relatively mild conditions should be employed, and special attention conditions should be utilized when conducting the extraction. Therefore, different separation techniques, e.g., thin layer chromatography (TLC), column chromatography (CC), low pressure liquid chromatography (LPLC), medium pressure liquid chromatography (MPLC), and high-performance liquid chromatography (HPLC), are typically used to achieve pure saponin components21.
Centrifugal partition chromatography (CPC) is known as liquid–liquid partition chromatography without any sorbent, which increases sample recovery and eliminates peak tailing on solid supports22. Thanks to these two advances, CPC has been accomplished using the separation of several bioactive saponins. In 2011, Jing et al. applied centrifugal partition chromatography combined with evaporative light scattering detection (CPC-ELSD) for systematic separation and purification of four saponins, including notoginsenoside R, ginsenoside Rg, Re, and Rb from the crude extract in a one-step separation, of which purities were over 98% from . In 2013, Wang et al. successfully isolated and purified two ginsenosides, Rb (95.6%) and Rb (97.8%), from the extract of by high-performance centrifugal partition chromatography (HPCPC)24. In 2014, Bahrami et al. performed HPCPC-ELSD to purify the saponin mixture and to isolate saponin congeners and isomeric saponins from the extract of the viscera of sea cucumber .
α-Hederin is a bioactive triterpene saponin of leaves. Therefore, the extraction and isolation of α-hederin by traditional methods presents many difficulties. In this research, by applying the CPC technique, highly purified α-hederin (95.7%) was isolated in just one run, and the isolation yield compared to the crude extract was 12.7%. The results demonstrated that CPC was a feasible and efficient technique for the isolation and purification of α-hederin from leaves.
CONCLUSION
CPC proved to be an excellent method for separating α-hederin from leaves. In a single CPC run, a high purity of isolated α-hederin (95.7%) was obtained. This study could serve as a benchmark for the large-scale isolation of high-purity α-hederin from leaves.
LIST OF ABBREVIATIONS
ACN: acetonitrile
CC: column chromatography
CHCl: chloroform
COSY: correlated spectroscopy
DEPT: distortionless enhancement by polarization transfer
DMSO: dimethyl sulfoxide
ELSD: evaporative light scattering detection
EtOAc: ethyl acetate
HMBC: heteronuclear multiple bond correlation
HPCPC: high-performance centrifugal partition chromatography
HPLC-DAD: high-performance liquid chromatography-diode array detector
HSQC: heteronuclear single quantum coherence
LPLC: low pressure liquid chromatography
MeOH: methanol
MPLC: medium pressure liquid chromatography
NMR: nuclear magnetic resonance
TLC: thin layer chromatography
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
This research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.01-2019.28.
Author’s Contributions
Tran Phan Huynh Nhu: analyzed the data and contributed to the writing of the manuscript. Tran Chieu An: performed HPLC analyses and contributed to the writing of the manuscript. Pham Hong Ngoc: synthesized documents, analyzed the results, and proposed the outline for the article. Doan Le Quynh Nhu: extracted the sample and determined a-hederin structure by NMR spectroscopy. Pham Thi Thanh Lan: performed CPC run. Le Ngoc Hung: devised the project and the conceptual ideas. Nguyen Tuan Hiep: collected the sample. Phung Van Trung: devised the main conceptual ideas and developed the theoretical framework. All authors read and approved the final manuscript.

