Effect of reaction conditions on the etherification between glycerol and tert-butyl alcohol over beta zeolite
- Faculty of Chemical Engineering, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City, Vietnam
- Vietnam National University, Ho Chi Minh City, Vietnam
- Department of Chemical Engineering, School of Chemical and Environmental Engineering, International University, Ho Chi Minh City, Vietnam
- NTT Hi-tech Institute, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam
- Hong Bang International University, Ho Chi Minh City, Vietnam
Abstract
Introduction: Glycerol tert-butyl ethers (GTBEs) can be used as diesel additives due to their physico-chemical properties, such as flash point, viscosity, and cetane number. GTBEs were produced from the reaction between tert-butyl alcohol and glycerol using a stirred reactor at moderate temperatures. This study aims to discuss the effect of reaction conditions on the etherification between glycerol and tert-butyl alcohol.
Methods: In this work, the etherification of glycerol and tert-butyl alcohol was performed using beta zeolite (SiO2/Al2O3 = 360:1) under different reaction conditions, including different molar ratios, catalyst loadings, reaction times, and reaction temperatures, to evaluate glycerol conversion and product selectivity. Beta zeolite was characterized by infrared spectroscopy, X-ray diffraction, and NH3-TPD.
Results: The results showed that the suitable conditions for the synthesis of GTBEs using beta zeolite were a temperature of 80 °C, a tert-butyl alcohol/glycerol feed ratio of 12:1, a catalyst loading of 1:3 weight of glycerol, and a reaction time of 6 hours. Under these conditions, the highest glycerol conversion was 81.35%, the selectivity of the diether was 32.44%, and the overall yield of the diether was 26.39%.
Conclusion: Reaction conditions are important factors in tailoring glycerol conversion and product selectivity in the reaction between glycerol and tert-butyl alcohol.
Introduction
The development of industries is driving an increase in energy demand. Diesel, a vital energy source for transportation, power generation, construction, manufacturing, and farming, is predominantly derived from petroleum resources. However, an alternative source, biodiesel, can be produced from green resources through transesterification of glycerides with alcohols 1, 2. This process also yields glycerol as a byproduct. The growing demand for bioenergy, including biodiesel, has led to a surge in glycerol production. Consequently, there is significant interest in converting glycerol into valuable products, particularly within the biodiesel industry.
Currently, glycerol can be converted into several products, including syngas, solketal, glycerol carbonate, glyceric acid, tartonic acid, propanediols, acrolein, and glycerol ether3, 4. Among these, ethers of glycerol, such as di-tert-butyl glycerol ethers and tri-tert-butyl glycerol, can be used as diesel additives3, 5. Several studies have explored the etherification of glycerol with tert-butanol 6, 7, 8, 9. Five main products, including two mono-tert-butylglycerol ethers (monoethers), two di-tert-butyl-glycerol ethers (diethers) and one tri-tert-butylglycerol (triether), were obtained from glycerol etherification with tert-butyl alcohol (Figure 1).

The scheme of glycerol etherification with tert-butanol
Acid catalysts, including Amberlyst 6, Y zeolite8, 10, FAU, MOR, MFI, Beta zeolites 10, 11, 12, and tungstophosphoric acid7, 13, were investigated. Amberlyst-15 showed high conversion of glycerol (at 90-100 °C) because of the high number of Brønsted acid sites 6. Among Beta, Faujasite, MFI and Mordenite zeolites, Pariente et al10 reported that Beta zeolite with an intermediate aluminum content (Si/Al = 25) is effective for glycerol etherification with ethanol. Beta zeolite with Si/Al = 25 showed a higher glycerol conversion and diether selectivity among the investigated zeolites 10. Specifically, nanobbeta zeolite (SiO/AlO = 25) resulted in ~95% glycerol conversion and ~99% glycerol conversion toward diethers and triethers11. In this research, we studied the etherification of glycerol with tert-butanol using a beta zeolite with a high Si/Al ratio that typically contains mainly Brønsted acid sites and explored the effects of the reaction conditions on this etherification reaction.
Materials and Methods
Materials
Commercial beta zeolites with a SiO/AlO ratio of 360 were purchased from Alfa Aesar and denoted as beta 360. Glycerol (Fisher, 99%), ethanol (Fisher, 99.8%) and tert-butyl alcohol (Shanghai Zhanyun Chemical Co., Ltd., AR grade) were used without any pretreatment. Dodecane (Fisher, 95%) was used as an internal standard.
Catalyst characterization
The ATR-FT-IR spectra of the zeolite samples were characterized by a Bruker VERTEX70 instrument coupled with a Platinum Diamond ATR in the wavenumber range of 400-4000 cm, with a resolution of 4 cm and an accumulation of 32 scans. The sample was placed on the ATR crystal, and the spectrum was recorded in air. The background spectrum was taken before sample analysis. The catalyst surface was analyzed by an X Shimadzu 6100 (Japan) instrument operating at a voltage of 40 kV and a current of 30 mA with CuK radiation at a wavelength of 0.15406 nm. The 2θ ranged from 10 to 60, with each step being 0.02, and the scanning speed was 0.05/sec. The sample was powder and placed in the sample holder. The ammonia-temperature programmed desorption (NH-TPD) profile was measured as described in a previous study 14. The NH-TPD profile was measured using a calorimeter (Sensys DSC, SETARAM instrument, Cranbury, NJ, USA). After pelletizing and collecting particles ranging from 180 to 250 µm, the sample (30 mg) was pretreated at 300 °C for 30 min in Ar and then cooled to 100 °C. The sample was saturated with NH by a flow of 20 mL/min containing 2000 ppm NH balanced in Ar for 90 min. After that, the physisorbed NH was removed from the sample by flushing with Ar for 60 min. Temperature-programmed desorption measurements were performed by increasing the temperature from 100 to 700 °C (heating rate 10 °C/min). The mass number, m/z = 17, was recorded to follow the desorption of NH using a mass spectrometer (HPR-20 QIC, Hidden Analytical, Warrington, UK).
Glycerol etherification reaction
A 100 cm glass batch reactor was used to carry out the liquid phase etherification process between glycerol and tert-butyl alcohol at a stirring frequency of 600 cycles per minute. To maintain a consistent temperature in the glass, the reactor was placed in a water bath. The experiments were performed under different reaction conditions: (i) tert-butyl alcohol/glycerol (TBA/G) molar ratio; (ii) reaction time (iii) reaction temperature ranging from 60 °C to 90 °C; and (iv) catalyst loading (0.045–0.45 g). The experimental procedure involved adding glycerol (0.45 g), catalysts and tert-butyl alcohol to the reactor. n-Dodecane was also added to 10 wt.% glycerol as an internal standard for subsequent sample analysis. The reactor was then sealed and stirred at a constant temperature. The reaction was stopped by immersing the reactor in a cold-water bath to allow all of the gas phase chemicals to condense into a completely liquid mixture. Approximately 2 mL of ethanol was added to the mixture to homogenize the remaining glycerol with tert-butyl alcohol. The product was then centrifuged at 8000 revolutions per minute for 10 minutes to remove the solid catalysts. The liquid sample was diluted with ethanol before analysis. All reactions were performed in triplicate.
All products that were diluted in ethanol and aliquoted were subsequently analyzed for glycerol conversion, diethers and tri-ether selectivity by gas chromatography (GC). The liquid mixture was analyzed using an Agilent 7890A gas chromatography (GC) instrument equipped with a flame ionization detector (FID) for product quantification. An automatic liquid sampler (ALS) G4513A automatic liquid sampler (ALS) Agilent 7693A ALS with G4513A Injector was used, and 1 μL of sample was injected at a split ratio of 40:1. An HP-5 capillary column (length, 30 m; inner diameter, 0.32 mm; film thickness, 0.25 μm) was used for product separation under the following oven temperature profile: from 45 °C to 85 °C (with a heating rate of 10 °C/min), held for 2 minutes, then heated to 250 °C and held for 2 minutes.
The glycerol conversion and product selectivity were calculated as follows:
The conversion of glycerol =
The selectivity of monoether =
The selectivity of diether =
The yield of ether = Glycerol conversion (%) × ether selectivity (%)
n: initial mole of glycerol (mol).
n: mole of glycerol remaining after reaction (mol).
∑ A : total area of monoether peaks from GC data.
∑ A : total area of diether peaks from GC data.
∑ A: total area of all glycerol tert-butyl ether peaks from GC data.
To determine the peak of each compound, the products were analyzed by gas chromatography–mass spectrometry (GC–MS) on an Agilent 6890 instrument equipped with a 5973 inert mass selective detector (MSD). An injector was used to inject 1 μL of sample at a split ratio of 30:1. A GSBP-5MS capillary column (length, 30 m; inner diameter, 0.25 mm; film thickness, 0.25 μm) was used for product separation under the following oven temperature profile with a starting point at 60 °C and was held for 4 minutes. The mixture was heated to 90 °C at a heating rate of 10 °C/min, held for 1 minute, then continuously heated to 150 °C and held for 5 minutes. GC‒MS analysis, as shown in Figure 2 revealed that the glycerol etherification reaction with tert-butyl alcohol produced monoether and diether and did not produce triether in this study using beta 360 zeolite.

GC‒MS data of glycerol etherified with tert-butyl alcohol over beta 360 zeolite. The data show the presence of mono-tert-butyl-glycerol ethers (monoethers) and di-tert-butyl-glycerol ethers (diethers).
Results
Characterization of the catalyst
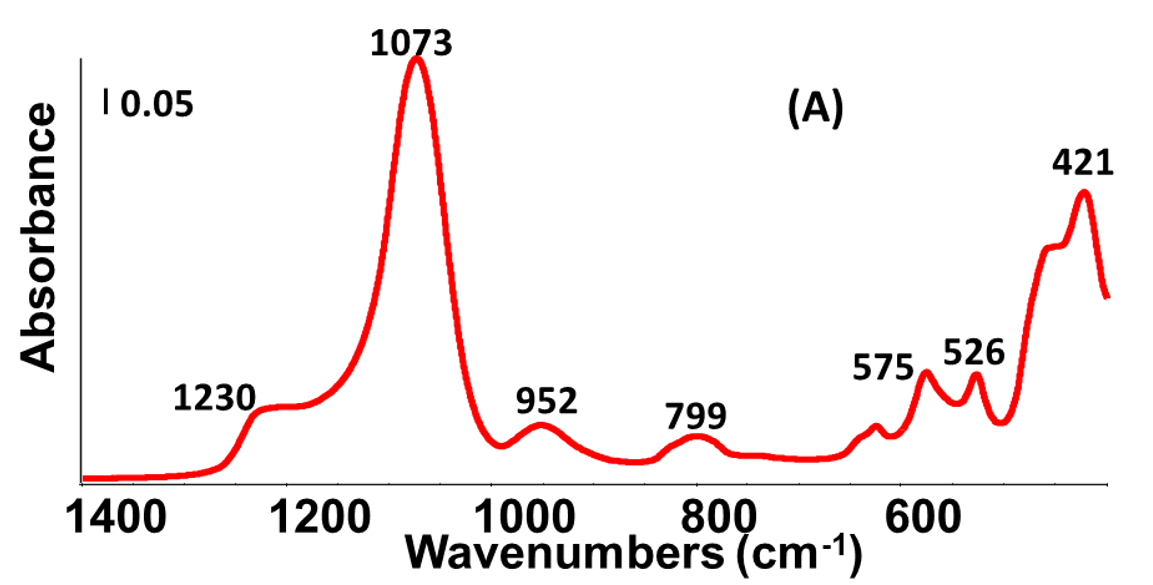
Figure 3A shows that beta 360 presented three main peaks at 1300–900 cm, 850–650 cm, and 465–400 cm15. The peaks in the range of 1300–900 cm are assigned to the asymmetric stretching of the T–O–T bridges, where T represents tetrahedrally coordinated Si or Al atoms. There are three peaks in this range at 1230, 1073, and 952 cm. The peak at 799 cm is assigned to the bending mode of the T–O–T bridges. The last range, which represents the out-of-plane deformation of the T–O–T bridges, contains several peaks at 575 cm, 526 cm, and 421 cm15.

(A) FT-IR spectrum of beta 360 zeolite; (B) XRD pattern of beta 360 zeolite with two main peaks at 2θ values of 7.9° and 22.6°.
Figure 3B presents the XRD pattern of the beta 360 zeolite. The XRD pattern showed two main peaks at 2θ values of 7.9° and 22.6°, indicating the presence of the crystallized structure of the beta zeolite. This finding agrees with the JCPDS 48–0074 standard and with a previous study16. Moreover, the NH-TPD profile confirmed the existence of acid sites on the Beta 360 zeolite with two peaks at ~ 350 °C and ~ 600 °C in the temperature program desorption of NH, and the total acid density was 294 μmol NH/g catalyst (Figure 4).

NH3-TPD profile of the beta 360 zeolite shows the presence of acid sites with two main peaks at ~ 350 °C and ~ 600 °C.
Glycerol etherification studies

The conversion of glycerol and selectivity of monoether and diether in the etherification glycerol with tert-butyl alcohol as a function of (A) the tert-butyl alcohol/glycerol molar ratio. Reaction conditions: 0.45 g glycerol, 0.15 g catalyst, 80 °C, 6 h; (B) reaction time. Reaction conditions: 0.45 g glycerol, 0.15 g catalyst, 80 °C, TBA/G = 12:1; (C) reaction temperature. Reaction conditions: 0.45 g glycerol, 0.15 g catalyst, 6 h, TBA/G = 12:1; (D) amount of catalyst loading. Reaction conditions: 0.45 g glycerol, 80 °C, 6 h, TBA/G = 12:1
Glycerol conversion increased with an increase in the TBA/G ratio from 3:1 to 12:1 and decreased thereafter (Figure 5A). Glycerol conversion reached its highest value of 81.35% at a TBA/G ratio of 12:1, and at this TBA/G ratio, the selectivity for diether production also peaked at 32.44%, indicating that the yield of diether was 26.39%. This phenomenon can be explained by the reaction mechanism17, 18. An increase in the tert-butyl alcohol to glycerol (TBA/G) ratio increases the amount of [(CH)C] species, enhancing the reaction between glycerol and [(CH)C] species and thus increasing glycerol conversion. However, at a higher TBA/G ratio (15:1), the high concentration of TBA decreases the glycerol concentration, reducing the interaction of glycerol with the limited reactive surface area of the catalyst19, 20 and resulting in decreased glycerol conversion.
In terms of reaction time, an increase in reaction time enhances glycerol conversion from 2 hours to 6 hours and decreases it after 8 hours of reaction (Figure 5B). An increased reaction time promoted the reaction between glycerol and tert-butyl alcohol, leading to increased glycerol conversion. However, at longer reaction times (8 h), tert-butyl alcohol possibly tends to dehydrate to form iso-butylene, followed by the polymerization of iso-butylene7, which reduces the catalytic activity and selectivity for diether production.
In terms of reaction temperature, both glycerol conversion and diether selectivity increased as the temperature increased from 60 to 80 °C (Figure 5C). However, at 90 °C, both glycerol conversion and diether selectivity decreased. Indeed, the increase in temperature accelerated the collision between TBA and glycerol, enhancing the catalytic activity. However, at 90 °C, side reactions such as ether hydration and TBA dehydration can occur, leading to decreased catalytic conversion7, 19. Ethers produced from the etherification of glycerol with tert-butyl alcohol can react with the water produced to regenerate glycerol and tert-butyl alcohol. Additionally, tert-butyl alcohol can dehydrate to form iso-butylene, reducing the amount of TBA relative to glycerol. Consequently, the catalytic activity decreases at higher temperatures (90 °C).
In addition to the effect of the reactant molar ratio, temperature, and reaction time, the amount of catalyst loading can also affect the conversion and selectivity of the products. Figure 5D shows that the highest glycerol conversion was achieved with 0.15 g of the beta 360 catalyst. The glycerol conversion increased from 0.045 g of beta 360 catalyst to 0.15 g of beta 360 catalyst and then decreased from 0.15 g to 0.45 g of catalyst (Figure 5D). Moreover, the selectivity of diether reached its peak with 0.15 g of catalyst and remained relatively constant when 0.15 g to 0.45 g of catalyst was used. An increase in catalyst loading (0.045 g to 0.15 g of catalyst) meant that an increase in acid density enhanced the formation of [(CH)C] species from tert-butyl alcohol, thereby improving the catalytic activity and product distribution. However, a higher catalyst loading (0.3 g to 0.45 g) leads to too high an acid density, which tends to dehydrate TBA, causing a decrease in the TBA/G ratio7, 19 and, consequently, a decrease in glycerol conversion.
Discussion
The characteristics of the catalyst were confirmed by IR spectroscopy and XRD, which demonstrated that the catalyst used for this study was a beta zeolite (Figure 3). The results also showed that the beta 360 zeolite contains acid sites, as proven by NH-TPD analysis (Figure 4), making it suitable for glycerol etherification with tert-butyl alcohol17, 18. Indeed, [(CH)C] species were formed on the surface of the acid sites of beta 360 zeolite17. Then, an intermediate will be formed between the [(CH)C] cation species and glycerol, followed by the liberation of acid sites to create a monoether17. The diether and triether can be formed in the same manner as the interaction between the [(CH)C] species and the monoether and diether, respectively, on the surface of acid sites. This indicated that the acid sites of the beta zeolite were important active sites for the glycerol etherification reaction with tert-butyl alcohol. However, the type of acid sites on the catalyst can be explored by pyridine adsorption using IR spectroscopy. The reaction conditions for achieving the highest glycerol conversion and diether yield were 80 °C, TBA/G = 12:1, 6 h of reaction time, and 0.15 g of catalyst. Temperature is one of the key factors for glycerol etherification with tert-butyl alcohol. Higher temperatures enhanced the catalytic activity, but tert-butyl alcohol can dehydrate at very high temperatures, leading to a decrease in the TBA/G ratio followed by a decrease in glycerol conversion. The TBA/G ratio and reaction time have strong impacts on glycerol conversion (Figure 5). The appropriate amount of tert-butyl alcohol used to react with glycerol was 12:1, and the reaction time was 6 hours. A prolonged temperature will enhance glycerol conversion, but ethers can decompose or transform into other products over a longer time. In addition, the amount of catalyst can also affect the catalytic activity and product selectivity. The amount of catalyst is relative to the amount of acid sites that catalyze tert-butyl alcohol. An appropriate amount of catalyst (0.15 g) resulted in the highest glycerol conversion. The glycerol conversion decreased when the amount of catalyst was too high (> 0.15 g) because side reactions could occur. These conditions can be further optimized through the use of experimental design to maximize glycerol conversion and diether selectivity. The diether yield (26.39%) in this study using beta 360 zeolite is comparable to the results obtained using beta zeolite with intermediate Si/Al ratios, such as h-Beta with Si/Al = 27.7 (di+triether yield = 26% at 75 °C and 4 h)21 and H-BETA with Si/Al = 25 (yield = 25.4% at 95 °C and 6 h) 22, and lower than the di- and triether yields (68.4% at 90 °C and 6 h) obtained using Nano-Beta zeolite23. However, the results of this study are promising when using beta zeolite with a very high Si/Al ratio at 80 °C. Additionally, the goal of the etherification of glycerol with tert-butyl alcohol is to produce diether and triether, which are diesel additives. However, monoether and diether were produced in this study. To produce triether with a large molecule, the pore size of beta zeolite may need to be increased to obtain both diether and triether.
Conclusions
This study investigated the effect of reaction conditions on glycerol etherification with tert-butyl alcohol over beta zeolites (SiO/AlO = 360). This work revealed that glycerol conversion and product selectivity depend on the reaction conditions. The glycerol conversion and diether selectivity increased with increasing reaction temperature from 60 to 80 °C and decreased at 90 °C. Similarly, they increased with an increase in the tert-butyl alcohol/glycerol molar ratio from 3:1 to 12:1 and decreased at a 15:1 ratio. The reaction time also affected glycerol conversion and product selectivity, with glycerol conversion and diether selectivity increasing from 2 hours to 6 hours of reaction time and decreasing at 8 hours of reaction. In addition, glycerol conversion and diether selectivity increased with increasing catalyst loading up to 0.15 g of catalyst and decreased at higher catalyst loadings (> 0.15 g). The highest glycerol conversion (81.35%) and diether selectivity (32.44%)/yield (26.39%) were obtained at 80 °C, TBA/G = 12:1, 6 h of reaction time, and 0.15 g of catalyst. This study can be further extended to produce both di-tert-butyl-glycerol ethers (diethers) and tri-tert-butylglycerol (triether), which can be used as diesel additives. Additionally, this study provides a background for research on the use of etherification to produce diesel additives under mild conditions.
LIST OF ABBREVIATIONS
ATR-FT-IR: Attenuated total reflectance - Fouriertransform infrared spectroscopy
G: Glycerol
GTBEs: Glycerol tert-butyl ethers
TBA: Tert-butyl alcohol
TBA/G: Tert-butyl alcohol to glycerol molar ratio
COMPETING INTERESTS
The author(s) declare that they have no competing interests.
ACKNOWLEDGMENT
This work is funded by Hong Bang International University under grant code GVTC17.52.
Author contributions
Hong-Gam Thi Nguyen: Writing – original draft, data curation and formal analysis
Phuc Hoang Dang: Data curation and formal analysis,
Thanh Ngoc Nguyen: Formal analysis
Thanh Khoa Phung: investigation, supervision, writing – review & editing
Tai Chiem Do: Funding acquisition, project administration, investigation, supervision, writing – review & editing

