Enhanced Absorption Capacity of Carboxymethyl Cellulose-Derived Superabsorbent Polymers: The Role of Acrylic Acid and Acrylamide Ratios
- Faculty of Chemistry, University of Science, VNU Hanoi, 19 Le Thanh Tong, Hoan Kiem, Hanoi
Abstract
Biodegradable superabsorbent polymer (BioSAP) synthesized from natural polymers has potential for agricultural use because of its three-dimensional hydrophilic network, which can absorb, swell, and retain a huge amount of water and salt solution. In this study, BioSAP was prepared by graft copolymerization of acrylic acid (AA) and acrylamide (AM) to the pineapple-leaf-derived car- boxymethyl cellulose using the inverse suspension method with different AA and AM ratios. The absorbent capacity and release performance of BioSAP for water and salt solution depend strongly on the AA/AM ratio. Among these samples, BioSAP with an AA/AM molar ratio of 6 exhibited the most stable gel structure and the maximum absorbencies for distilled water and salt solutions. This may be because AA can enhance the absorption and release ability of BioSAP, while AM can improve its gel stability. In addition, the presence of AA/AM in BioSAP enhances the water retention ability of the soil, leading to an increase in the storage time of water in the soil and improving the moisture content of the soil. These findings indicate that BioSAP synthesized with this AA/AM molar ratio is suitable for agricultural fields, improving soil moisture and helping plants grow better.
Introduction
Superabsorbent polymers (SAP) contain numerous ionic and/or hydrophilic groups in the polymeric network, which help them absorb and retain huge amounts of water up to several thousand times their dry weight1, 2, 3. Therefore, SAP can be widely applied in agricultural fields to decrease water consumption and increase crop yield4. SAP is typically mixed with soil, in which it absorbs water and swells after soil watering and releases water slowly when the soil dries. However, most conventional SAPs have been synthesized using petroleum-derived polymers, which are expensive, non-renewable resources and can lead to environmental pollution5. The use of natural polymers to prepare biodegradable superabsorbent polymers (BioSAP) is gaining more attention because they are abundant, inexpensive, non-toxic, and biodegradable6, 7, 8.
Cellulose and cellulose derivatives such as carboxymethyl cellulose (CMC) exhibit excellent water absorbency and can swell in water to form hydrogels9. These are promoted as alternative materials for SAP production. However, cellulose and cellulose-derived compounds are highly biodegradable in soil, meaning that they have short service lives, which limit their usefulness in agriculture. Grafting natural polymers with synthetic polymers is considered a promising method for overcoming this concern about BioSAP10, 11, 12.
Graft-copolymerization is considered a promising method for the synthesis of BioSAP with long cycle life and high biodegradability13, 14, 15, 16. Ismaeilimoghadam et al. produced BioSAP by grafting cellulose with acrylic acid (AA) to form a semi-interpenetrating structure hydrogel, which exhibited high water absorption capacities up to 142.29 g/g in 0.9% NaCl solution and 817.4 g/g in distilled water17. Besides, the combination of sodium alginate and AA forming an interpenetrating network can improve the mechanical properties of BioSAP. Wang et al. synthesized AA-grafted CMC and obtained the highest water absorbency of 750 and 85 g/g in distilled water and 0.9 wt.% NaCl solution, respectively18. The combination of different natural and synthetic polymers leads to the ampleness and diversity in the polymeric structure of BioSAP, directly resulting in their absorption-desorption performance.
In this study, BioSAP materials were fabricated from pineapple-leaf-derived CMC, AA, and acrylamide (AM) using a chemical graft method to improve their water absorbency in salt solutions and for soil-conditioning applications. The effects of AA and AM monomers on the water absorption–desorption performance of BioSAP were investigated. The water absorption–desorption properties of BioSAP in water, salt solution, and soil changed drastically depending on the AA/AM ratio. The BioSAP with AA monomer exhibited high water absorption capacity and weaker mechanical stability, while the BioSAP with AM monomer exhibited stronger mechanical performance and lower absorption capacity. Controlling the contents of AA and AM monomers in the polymeric structure of BioSAP can produce BioSAPs with various absorption and mechanical properties for various applications.
Materials and Methods
CMC was prepared from pineapple leaves as reported in the literature19. N,N′-methylene bisacrylamide (MBA) 99% and ammonium persulfate (APS) 99% were purchased from Biobasic (Canada). AA 99%, AM 99%, and Span-60 98% were procured from Merck (Germany). Cyclohexane 98% and acetone 98% were obtained from China.
BioSAP was synthesized by graft copolymerization of AA and AM to the pineapple leaf-derived CMC with different AA and AM ratios (100 % AA, AA/AM = 9, AA/AM = 6, AA/AM = 3, and 100 % AM). First, the A solution was prepared by dissolving CMC into distilled water containing a certain amount of AA and AM monomers, followed by adding 0.1 wt.% MBA and 0.5 wt.% APS. The B solution was simultaneously prepared by mixing 1.5 wt.% Span-60 with cyclohexane, following by introducing N flow at 40 °C for 30 min. Second, the A solution was dropwise added into the B solution at 60 °C under vigorous stirring and N flow until the mixed solution became a concentrated gel. The mixture was maintained at 60 °C for the next 2 h. The gel product was cut into small pieces, soaked with ethanol overnight, and dried at 60 °C for 8 h. The gel was refined by the Soxhlet process in a mixture of acetone and water (volume ratio of 7:3 v/v) for 8 h, followed by drying and grinding to obtain BioSAP.
Fourier transform infrared spectroscopy (FTIR)
FTIR was conducted using an FTIR-6300 spectrometer (Jasco, Japan). The spectral resolution was 4 cm, and the absorption region was 600–4000 cm.
Mechanical stability measurement
Three cubic samples of each BioSAP gel were tested to measure their mechanical stability. The BioSAP samples were placed in water until they absorbed as much water as possible. A standard weight of 200 g was then placed on each gel cube. The state of each sample was observed before and after application of the weight.
Water absorption–desorption performance in solution
For all measurements, three samples were used, and the results were averaged.
The absorption performance of BioSAP was assessed by saturation for up to 2 days in solutions containing reverse osmosis (RO) water and NaCl, KCl, CaCl, or FeCl. A defined weight () of BioSAP was placed in the water or salt solution at room temperature. The weight of the swollen BioSAP was measured at specified times (). The absorption capacity ( g/g) of the BioSAP was calculated as follows:
Each completely swollen BioSAP sample was weighed () and placed in a glass container at room temperature. The weight of the sample was measured every day () for 9 days. The water retention ( %) was estimated as follows:
Reusability: 1 g of the BioSAP material was kept in the RO water until it reached saturation. The swollen sample was filtered and weighed to calculate the water absorption capacity. The sample was then dried, and the next cycle was conducted.
Water absorption–desorption performance in soil
The experiments were conducted on both dry soil (200 g) and a mixture of dry soil (200 g) and BioSAP (1 g). The samples were placed in a beaker and weighed (). RO water was slowly added to the samples until they reached equilibrium. The beaker was weighted again (). The water-holding ratio ( %) was calculated using Eq. (3):
All the samples were maintained at 25 ᵒC and weighed () after (h). The water retention capacity (WR %) on the soil was calculated using Eq. (4):
Results
The chemical structure of BioSAPs with different AA/AM ratios were investigated by FTIR analysis. The results are show in Figure 1. The absorption bands located at approximately 3342 cm, 2922 cm, and 1043 cm correspond to the stretching vibration of O–H, C–H, and -1,4-glycosidic bonds in the polymeric structure of cellulose, respectively20, 21. The two bands at 1560 and 1400 cm are due to the C=O stretching vibrations of the –COONa group20. The absorption bands at 1716 and 1670 cm are related to the C=O stretching vibrations of carboxyl groups and amide groups, respectively21. In addition, a band that appeared at 3186 cm can be attributed to the stretching vibration of the N-H bond22. It demonstrates that the BioSAP material was synthesized successfully with the presence of AA and AM monomers.
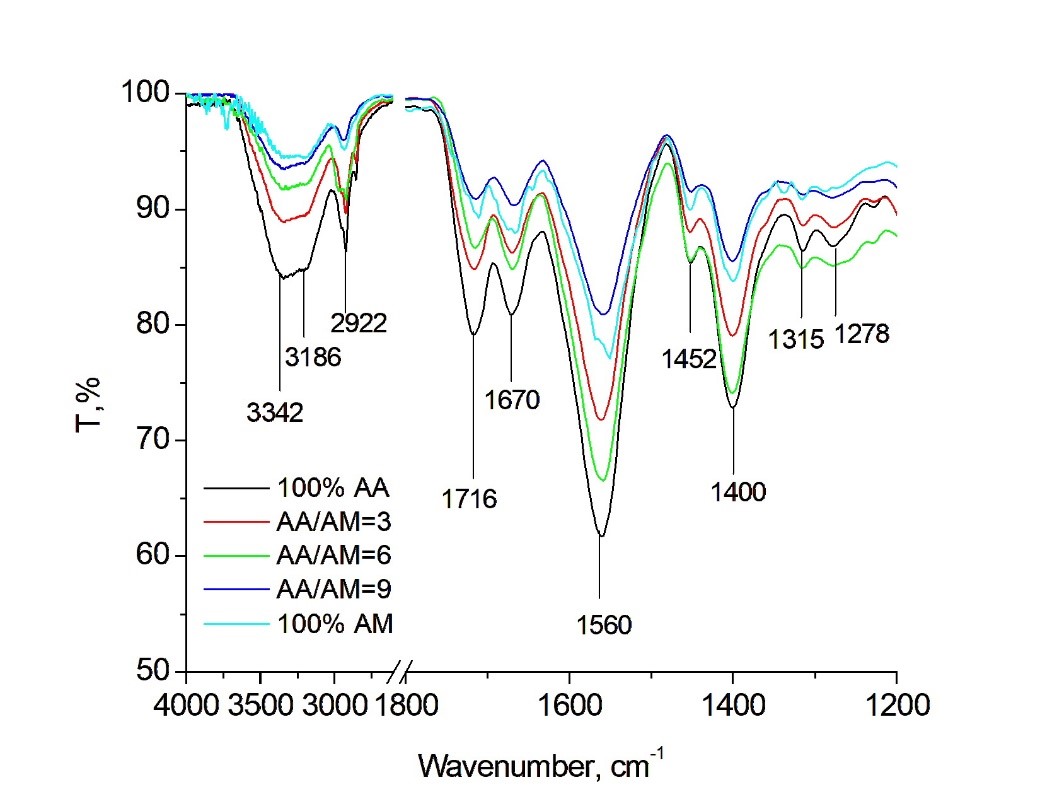
FT-IR spectra of the BioSAP sample with AA/AM =6
The mechanical stability of the BioSAP gel before and after measurement is illustrated in Figure 2. After absorbing water to equilibrium, BioSAP swells and changes to a gel. Sample cubes of BioSAP gel with different AM contents were loaded with standard 200-g weights to observe their mechanical responses. The stiffness of BioSAP gel increased with increasing AM monomer content. The BioSAP samples with a low AM content broke more easily into small pieces than those with a high AM content. These results indicate that AM monomer can improve the hardness and mechanical stability of BioSAP gel.
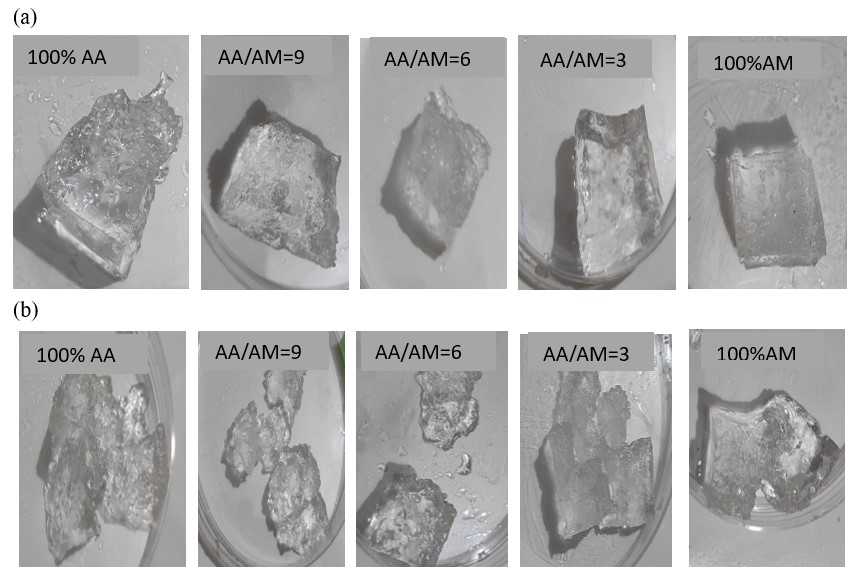
Physical photo of BioSAP with the different AA and AM content at the water saturation state (a) before and (b) after themechanical stability test
The as-synthesized BioSAP materials can absorb huge amounts of water and inorganic salt solutions such as NaCl, KCl, NHCl, FeCl, and CaCl. The water absorption and desorption activity of BioSAP are illustrated in Figure 3. All the BioSAP materials absorbed water rapidly, reaching a saturated state within 4 h. The contents of AA and AM monomers in the BioSAP strongly affected its water absorption and desorption performance. BioSAP containing only AA exhibited higher absorption capacity than BioSAP containing only AM (approximately 400 g/g and 40 g/g, respectively). It may come from the higher hydrophilicity of AA in comparison to AM, which facilitates the formation of linkage between BioSAP and water molecules more easily. The BioSAP with AA/AM = 6 exhibited the highest water absorption (approximately 1000 g/g). This indicates that an appropriate ratio of AA to AM monomers can enhance the absorption capacity of BioSAP.
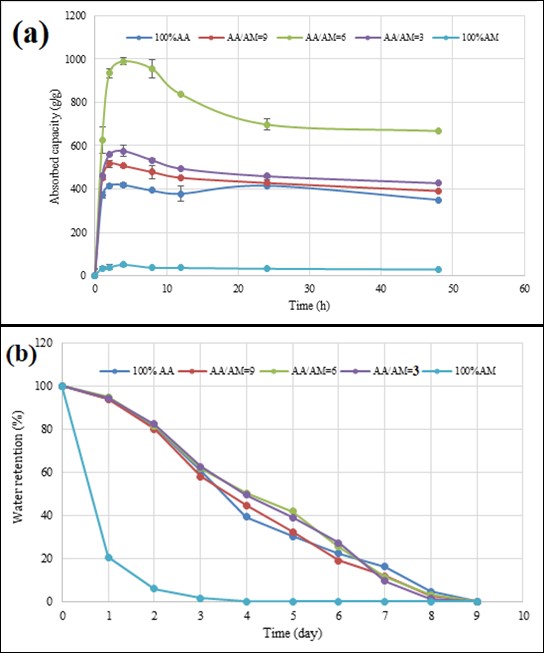
The RO water absorption (a) and desorption (b)performance of BioSAP with different AA/AM molar ratios
The water retention of BioSAP is shown in Figure 1(b). In general, the water retention capacity of these BioSAP samples decreased with increasing time. However, the water release activity of the different BioSAP samples varied because of their different contents of AA and AM monomers. The BioSAP with 100% AM quickly desorbed water in the first 2 days and released all the absorbed water within 4 days. In contrast, the BioSAP materials containing AA released water more slowly and exhibited a higher water retention capacity. The absorbed water molecules gradually left the polymer structure of these BioSAP materials within 9 days.
The reusability of the BioSAP materials is illustrated in Figure 4. The water absorption capacity was determined in the saturated state. BioSAP with the AA/AM = 6 exhibited the highest water absorption over the two cycles, as a result of the high hydrophilicity of the AA monomer. However, all the BioSAP samples show a decrease in water absorption capacity after two cycles. This may have been due to the collapse of the polymer network and crosslinking in the BioSAP after the water absorption and release processes.
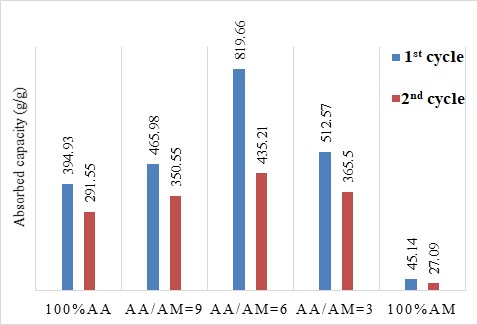
Reusability of BioSAP in RO water
The water absorption and desorption performance of BioSAP with AA/AM = 6 in the various salt solutions containing NaCl, KCl, NHCl, CaCl, and FeCl is displayed in Figure 5. The BioSAP samples need a longer time (12 h) to reach a saturated state. The water absorption capacities of BioSAP samples in salt solutions were much lower than those in RO water. This difference can be explained by the shielding effect of the cations in the salt solution, which inhibits electrostatic repulsion and shields the –COO. This shielding effect leads to a decrease in the osmotic pressure difference between the BioSAP and the salt solution, resulting in a lower water absorption capacity. The water absorption performance of BioSAP varies significantly depending on the cationic charge. BioSAP exhibits higher water absorption capacity in a solution with lower cationic charge and vice versa. The water absorption capacity decreases with the cation type as follows: Na > K > NH > Ca > Fe, which is consistent with reports in the literature2023.
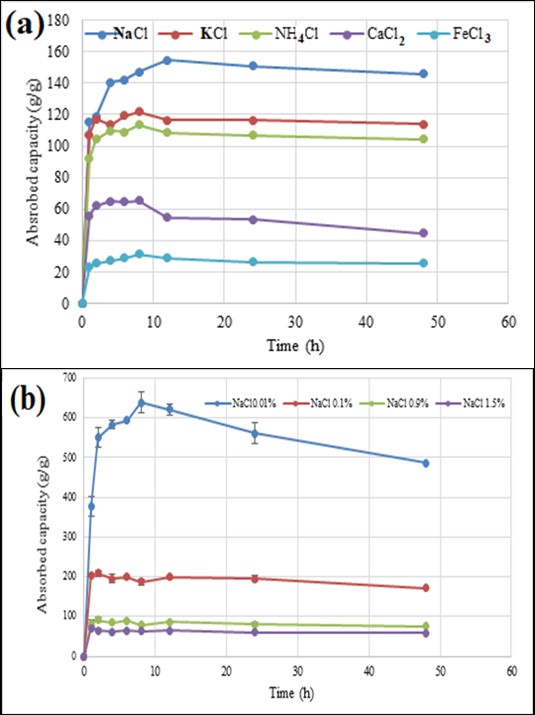
The water absorption performance of BioSAP withAA/AM = 6 in (a) salt absorption and (b) NaCl solution
The water absorption performance of BioSAP is dependent not only on the cationic charge but also on the concentration of the salt solution. The results shown in Figure 5(b) indicate that the water absorption capacity decreases with increasing salt concentration. The presence of excess cations in the solution at high concentrations enhances the shielding effect of the cations.
The water absorption capacity and water retention of BioSAP must be investigated in soil conditions before it can be utilized in agriculture. Figure 6 illustrates the water absorption performance of BioSAP in soil. It can be observed that the BioSAP possesses high water absorbency even in soil environments. Among these samples, the BioSAP with AA/AM = 6 exhibits the highest water absorption capacity (approximately 210 g/g). This means that these materials have potential for agricultural application.
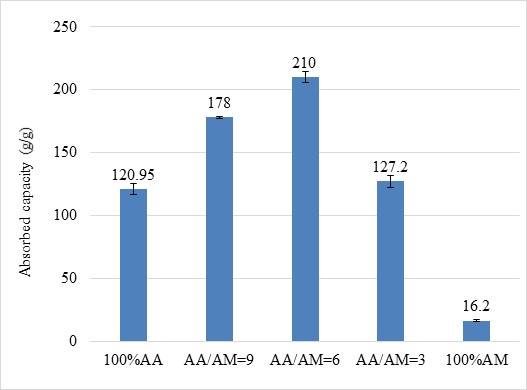
The water absorption in soil of BioSAP withdifferent AA and AM contents
The water retention capacity of BioSAP in the soil is shown in Figure 7. It can be seen that the soil mixed with BioSAP exhibits better water release performance than the soil without BioSAP. After 18 days, the soil without BioSAP lost all of its absorbed water, whereas the soil with BioSAP containing 100% AM still retained approximately 18% water. In contrast, the soil mixed with BioSAP containing AA monomer retained more than 40% of its absorbed water over the same period. These samples release the absorbed water completely within 40 days.
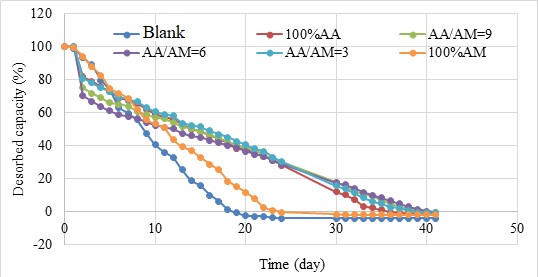
The water release activity of BioSAP in soil
Discussion
In this study, we prepared BioSAP materials using pineapple-leaf-derived CMC with the addition of AA and AM monomers. All the functional groups, which might be presence in BioSAP, are identified by IR measurement (Figure 1), indicating the successfully production of BioSAP with the AA and AM monomers. The mechanical properties and the absorption–desorption performance of as-synthesized BioSAP drastically changed depending on the ratio between AA and AM monomers in the polymer structure of BioSAP. AM monomer enhances the hardness and the mechanical stability of the BioSAP gel, shown in Figure 2.
BioSAP is considered a material with interesting potential applications because of its water absorption and slow release properties. The proportion of AA to AM monomers in BioSAP strongly influences the water and salt absorption–desorption performance of BioSAP. AA monomers contain a large number of strongly hydrophilic –COO groups, which can be easily linked to HO molecules, increasing the amount of water absorbed. As a result, AA monomers improve the water absorption of BioSAP. In contrast, the –CONH groups of AM monomers have weak hydrophilicity, leading to low absorption performance. In this study, BioSAP with only AA exhibited a high water absorption capacity, making the gel softer, moister, and weaker after absorption, resulting in low mechanical stability. A combination of AA and AM monomers in BioSAP can improve both its absorption–desorption performance and its mechanical stability.
With respect to desorption, the presence of AA monomer in the structure of BioSAP improves the interaction of hydrogen bonding and Van der Waals forces between HO molecules and BioSAP. The water retention capacity of BioSAP is increased as a result. BioSAP can retain water in its structure and release it over time at a rate that is suitable for agricultural applications, in which water and salt absorption and release must be controlled. The additive monomer content is the key factor in the absorption performance of BioSAP.
The BioSAP materials examined in this study exhibited good absorption–desorption performance in a soil environment. BioSAP can absorb and retain water and salt in its polymer network and then gradually release them to the surrounding soil over 18 days. The absorption capacity and slow release behavior of BioSAP indicate that mixing it with soil can enhance not only the water absorption capacity but also the water retention capacity of the soil, which would be beneficial for crop growth and reduced water consumption.
Conclusions
BioSAP was synthesized successfully via the chemical grafting method using pineapple-leaf-derived CMC with different contents of AA and AM monomers. The synthesized BioSAP exhibited high water absorption capacity and slow release of water into soil. The water absorption capacity was up to 819.66 g/g for the BioSAP sample with AA/AM = 6. The results of this study demonstrate that the contents of AA and AM monomers in the polymeric network of BioSAP significantly influence the water absorption–desorption performance of BioSAP. In particular, the highly hydrophilic –COO of AA enhances the water absorbency of BioSAP, while the AM monomer can improve the stiffness and mechanical stability of BioSAP gel. The synergy between AA and AM monomers leads the BioSAP material to achieve a balance between the water absorption capacity, water retention, and mechanical stability. Thus, BioSAP synthesized as described in this article is suitable for use in agricultural fields.
List of abbreviations
BioSAP – biodegradable superabsorbent polymers
CMC – carboxymethyl cellulose
AA – acrylic acid
AM - acrylamide
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors' Contributions
Thi Huyen Ngo: Investigation, Methodology, Data curation. Hai Linh Nguyen: Data curation, Formal analysis. Thi Dao Phan: Data curation, Formal analysis. Thi Tuyet Mai Phan: Conceptualization, Project administration, Writing – review & editing. Tuan Ngoc Phan: Writing – original draft, review & editing, Supervision.

