Copper promoted, direct sulfenylation of n-aryl pyrazoles
- Faculty of Chemical Engineering, Ho Chi Minh City University of Technology (HCMUT), VNU-HCM, Ho Chi Minh City
- VNU-HCM Key Laboratory for Functional Organic Materials
Abstract
Direct functionalization of N-heterocycles often attracts substantial attention, as the targeted compounds find ubiquitous uses in many applications. Among common N-heterocycles, substituted pyrazoles are still challenging compounds for functionalization, though commonly useful in bio- and material-related studies. Herein, we report a general method for direct sulfenylation at the C4-H bonds of pyrazoles with diaryl disulfides. This transformation produces unsymmetrical thioethers, which often require the use of pre-functionalized starting materials and partners with unpleasant smells. Our reaction conditions involve the use of a CuCl2 catalyst, K2S2O8 oxidant, and CH3COOH as the solvent. A wide range of functionalities, including bromo, chloro, and nitro groups, were well-compatible. A possible mechanism, including electrophilic sulfenylation, was proposed.
INTRODUCTION
Substituted pyrazoles represent a significant nucleus found in a wide range of medicinally relevant molecules. Celecoxib, perhaps, is the best-known drug derived from a substituted pyrazole1. Methods for the transition metal-catalyzed, pyrazole-directed functionalization of C−H bonds have been extensively studied2, 3, 4, 5. Consequently, substantial efforts have been devoted to developing methods for the functionalization and diversification of substituted pyrazoles6. Directed arylation, alkenylation, and alkynylation of C−H bonds in pyrazoles are known7, 8, 9. However, only a few methods for the direct functionalization of pyrazoles with heteroatom-based nucleophiles have been reported.
Sulfenylated pyrazoles could be used in several applications. For example, Fipronil, a phenylpyrazole-based insecticide, was obtained from the oxidation of a sulfenylated pyrazole10. A method for the synthesis of a sulfenylation analogue of the anti-inflammatory drug celecoxib was also reported11. Sulfenyl-substituted pyrazoles are often obtained via the sulfenylation of pyrazolones12, 13, 14. The transformation presumably starts with the tautomerization of pyrazolones to afford hydroxyl pyrazoles, followed by functionalization with sulfur-based nucleophiles assisted by the hydroxyl directing group. The approach was arguably limited by the scope of pyrazole substrates, as the presence of hydroxyl groups at the C5 positions was crucial for successful sulfenylation. Notably, only one example of direct functionalization of C4−H bonds in pyrazoles with aryl thiols has been reported15. Given the unpleasant smell of aryl thiols and the limited scope of substrates, there is still an expectation for more general methods for the sulfenylation of C−H bonds in pyrazoles.
In this study, we report a general method for the functionalization of C4−H bonds in pyrazoles with diaryl disulfides. The reactions progressed in the presence of a copper(II) chloride catalyst, K2S2O8 as the oxidant, and acetic acid as the solvent. The conditions were tolerant of functionalities, including bromo, chloro, and nitro groups. Our method would offer a convenient pathway to obtain sterically hindered N-aryl substituted pyrazoles from cheap, stable reagents.
MATERIALS-METHODS
Materials
Derivatives of 3,5-dimethyl-1-phenyl-1-pyrazole were prepared via condensation of arylhydrazine hydrochloride and acetylacetone following the known procedure reported by Daugulis and co-workers16. Other reagents, including diaryl disulfides, were commercially available and used as obtained without further treatment.
Characterization
Gas chromatography (GC) analyses were performed on a Shimadzu GC2010-Plus instrument equipped with a flame ionization detector (FID) and a SPB-5 column. Gas chromatography – mass spectrometry (GC-MS) analyses were performed on a Shimadzu GCMS-QP2010 Ultra instrument equipped with a ZB-5MS column. Proton and carbon-13 nuclear magnetic resonance (H NMR and C NMR) spectra were recorded on a Bruker AV 600 spectrometer.
General procedure for synthesis of 3,5-dimethyl-1-aryl-1-pyrazoles
To a 12-mL vial equipped with a magnetic stirrer was added an arylhydrazine hydrochloride (2 mmol), acetylacetone (2 mmol, 200.2 mg), and ethanol (2 mL). The mixture was stirred at 80 °C for 3 h. The crude reaction mixture was quenched with brine, then extracted with ethyl acetate (5 mL). The organic layer was washed with water (5 mL), dried over NaSO, filtered and concentrated. The crude 3,5-dimethyl-1-aryl-1-pyrazole was pure enough for further sulfenylation without purification.
General procedure for copper-catalyzed C4-sulfenylation of -aryl pyrazoles
To a 12-mL vial equipped with a magnetic stirrer was added 3,5-dimethyl-1-phenyl-1-pyrazole 1a or a derivative (0.1 mmol), diphenyl disulfide 2a or derivative (0.2 mmol), CuCl (0.02 mmol, 2.7 mg), KSO (0.15 mmol, 40.5 mg), and CHCOOH (1 mL). The mixture was stirred at 140 °C for 3 h. The crude reaction mixture was then quenched with NaHCO (10 wt% aqueous solution, 5 mL). Organic components were extracted with ethyl acetate (3 x 5 mL). Combined organic layers were dried over NaSO, filtered, concentrated, and diphenyl ether (0.1 mmol) was added as an internal standard. Analysis with GC was used to determine GC yields. Otherwise, purification by column chromatography using eluent toluene/ethyl acetate 50:1 would afford the sulfenylation product.

Direct sulfenylation of pyrazole C−H bond.

Scope of substrates regarding sulfenylation of pyrazole C−H bonds.

Control experiments.

Possible mechanism for sulfenylation.
RESULTS
The sulfenylation of 3,5-dimethyl-1-phenyl-1-pyrazole 1a with diphenyl disulfide 2a was studied with respect to the effect of copper salt, oxidant, and solvent (Figure 1). The results are presented in
Study of conditions for sulfenylation
|
Entry |
[Cu] |
oxidant |
solvent |
yield of 3aa (%) |
|
1 |
CuCl |
K2S2O8 |
CH3COOH |
40 |
|
2 |
CuCl2 |
K2S2O8 |
CH3COOH |
74 |
|
3 |
Cu(OAc)2 |
K2S2O8 |
CH3COOH |
20 |
|
4 |
CuBr2 |
K2S2O8 |
CH3COOH |
50 |
|
5 |
CuCl2 |
K2S2O8 |
DMSO |
trace |
|
6 |
CuCl2 |
K2S2O8 |
DMF |
trace |
|
7 |
CuCl2 |
K2S2O8 |
o-dichlorobenzene |
46 |
|
8 |
CuCl2 |
TBHP |
CH3COOH |
22 |
|
9 |
CuCl2 |
none |
CH3COOH |
trace |
With the conditions in hand, we next study the scope of substrates. The results are presented in Figure 2. A pair of control experiments were carried out, and the results are presented in Figure 3. A possible mechanism for this sulfenylation was shown in Figure 4. It should be noted that all compounds were carefully characterized by H and C nuclear magnetic resonance. The results are as follows:
3,5-Dimethyl-1-phenyl-4-(phenylthio)-1-pyrazole (3aa): yellow oil. H NMR (600 MHz, CDCl) δ 7.53 – 7.45 (m, 3H), 7.43 – 7.35 (m, 1H), 7.27 – 7.19 (m, 2H), 7.13 – 7.03 (m, 2H), 2.34 (d, = 0.7 Hz, 2H), 2.29 (d, = 0.7 Hz, 2H). C NMR (150 MHz, CDCl) δ 153.2, 144.1, 139.8, 138.3, 129.1, 128.9, 127.8, 125.4, 124.9, 124.7, 106.1, 12.0, 11.5.
3,5-Dimethyl-4-(phenylthio)-1-(-tolyl)-1-pyrazole (3ba): yellow oil. H NMR (600 MHz, CDCl) δ 7.37 – 7.32 (m, 2H), 7.29 – 7.26 (m, 2H), 7.24 – 7.20 (m, 2H), 7.12 – 7.07 (m, 1H), 7.07 – 7.03 (m, 2H), 2.41 (s, 3H), 2.31 (s, 3H), 2.28 (s, 3H). C NMR (150 MHz, CDCl) δ 153.0, 144.1, 138.4, 137.8, 137.3, 129.7, 128.8, 125.4, 124.8, 124.6, 105.7, 21.1, 12.0, 11.4.
1-(4-Methoxyphenyl)-3,5-dimethyl-4-(phenylthio)-1-pyrazole (3ca): yellow oil. H NMR (600 MHz, CDCl) δ 7.40 – 7.34 (m, 2H), 7.26 – 7.19 (m, 2H), 7.13 – 7.06 (m, 1H), 7.08 – 7.03 (m, 2H), 7.01 – 6.96 (m, 2H), 3.86 (s, 3H), 2.28 (s, 3H), 2.27 (s, 3H). C NMR (150 MHz, CDCl) δ 159.2, 152.8, 144.2, 138.4, 132.9, 128.8, 126.3, 125.4, 124.8, 114.3, 105.4, 55.5, 12.0, 11.3.
1-(4-Bromophenyl)-3,5-dimethyl-4-(phenylthio)-1-pyrazole (3da): yellow oil. H NMR (600 MHz, CDCl) δ 7.64 – 7.58 (m, 2H), 7.40 – 7.34 (m, 2H), 7.28 – 7.20 (m, 2H), 7.10 (td, = 7.5, 1.0 Hz, 1H), 7.07 – 7.02 (m, 2H), 2.34 (s, 3H), 2.28 (s, 3H).C NMR (150 MHz, CDCl) δ 153.6, 144.1, 138.8, 138.0, 132.3, 128.9, 126.0, 125.5, 125.0, 121.4, 106.9, 12.0, 11.6.
3,5-Dimethyl-1-(4-(methylsulfonyl)phenyl)-4-(phenylthio)-1-pyrazole (3ea): yellow oil. H NMR (600 MHz, CDCl) δ 8.11 – 8.05 (m, 2H), 7.79 – 7.73 (m, 2H), 7.26 – 7.22 (m, 2H), 7.12 (tt, = 7.0, 1.2 Hz, 1H), 7.08 – 7.04 (m, 2H), 3.10 (s, 3H), 2.46 (s, 3H), 2.29 (s, 3H). C NMR (125 MHz, CDCl) δ 154.5, 144.3, 144.0, 139.0, 137.5, 129.0, 128.7, 125.7, 125.3, 124.3, 108.7, 44.6, 12.13, 12.11.
1-(3-Methoxyphenyl)-3,5-dimethyl-4-(phenylthio)-1-pyrazole (3fa): yellow oil. H NMR (600 MHz, CDCl) δ 7.37 (t, = 8.3 Hz, 1H), 7.25 – 7.20 (m, 2H), 7.12 – 7.08 (m, 1H), 7.08 – 7.01 (m, 4H), 6.94 (ddd, = 8.3, 2.4, 1.0 Hz, 1H), 3.86 (s, 3H), 2.35 (s, 3H), 2.29 (s, 3H). C NMR (125 MHz, CDCl) δ 160.2, 153.1, 144.2, 140.8, 138.3, 129.8, 128.9, 125.4, 124.9, 116.8, 113.8, 110.4, 106.2, 55.5, 12.0, 11.6.
3,5-Dimethyl-1-phenyl-4-(-tolylthio)-1-pyrazole (3ab): yellow oil. H NMR (600 MHz, CDCl) δ 7.51 – 7.45 (m, 4H), 7.40 – 7.36 (m, 1H), 7.06 – 7.02 (m, 2H), 7.00 – 6.95 (m, 2H), 2.34 (s, 3H), 2.289 (s, 3H), 2.285 (s, 3H). C NMR (150 MHz, CDCl) δ 153.1, 143.9, 139.8, 134.8, 134.6, 129.6, 129.1, 127.7, 125.8, 124.7, 106.7, 20.8, 12.1, 11.5.
4-((4-Chlorophenyl)thio)-3,5-dimethyl-1-phenyl-1-pyrazole (3ac): yellow oil. H NMR (600 MHz, CDCl) δ 7.52 – 7.45 (m, 4H), 7.43 – 7.37 (m, 1H), 7.21 – 7.17 (m, 2H), 7.01 – 6.95 (m, 2H), 2.33 (s, 3H), 2.28 (s, 3H). C NMR (150 MHz, CDCl) δ 153.0, 144.1, 139.7, 136.9, 130.7, 129.2, 129.0, 127.9, 126.7, 124.7, 105.7, 12.0, 11.5.
3,5-Dimethyl-4-((3-nitrophenyl)thio)-1-phenyl-1-pyrazole (3ad): yellow oil. H NMR (600 MHz, CDCl) δ 7.94 (ddd, = 8.1, 2.0, 1.1 Hz, 1H), 7.88 (t, = 2.0 Hz, 1H), 7.53 – 7.47 (m, 4H), 7.45 – 7.38 (m, 2H), 7.34 (ddd, = 8.1, 2.0, 1.1 Hz, 1H), 2.35 (s, 3H), 2.29 (s, 3H). C NMR (150 MHz, CDCl) δ 153.0, 148.8, 144.5, 141.6, 139.5, 130.7, 129.6, 129.2, 128.1, 124.8, 119.85, 119.82, 104.2, 12.0, 11.5.
DISCUSSION
The results of studying reaction conditions showed that copper(I) chloride gave a moderate yield of 3aa (entry 1,
Isolation by column chromatography furnished 3aa in good yield (69%). Substitution on aryl rings attached to N1 atom of pyrazoles lowered the yield of the sulfenylation products. Moderate yields were obtained with pyrazoles derived from methyl- (3ba), methoxy- (3ca), and bromo- (3da) substituted arylhydrazines. Meanwhile, sulfenylation with the electron-rich bis(-methyl)phenyl disulfide afforded the product 3ab in good yield (65%). Electron-poor diaryl disulfides bearing chloro (3ac) and nitro (3ad) substituents could also be used as coupling partners, albeit affording the sulfenylation products in low yields (Scheme 2). For all substrates, sulfenylation always occurred selectively at C4 positions of pyrazole rings. Meanwhile, functionalization of C−H bonds on phenyl rings attached to N1 atoms was not observed, presumably due to the weak coordinating ability of pyrazole rings towards first-row transition metals such as copper salts. Methods for pyrazole-directed functionalization of C−H bonds on phenyl rings were only known for second-row transition metals16, 17. It should be emphasized that the use of such electron-poor substrate as the coupling partner for sulfenylation of C−H bonds in pyrazoles was limited in previous studies12, 13, 14, 15.
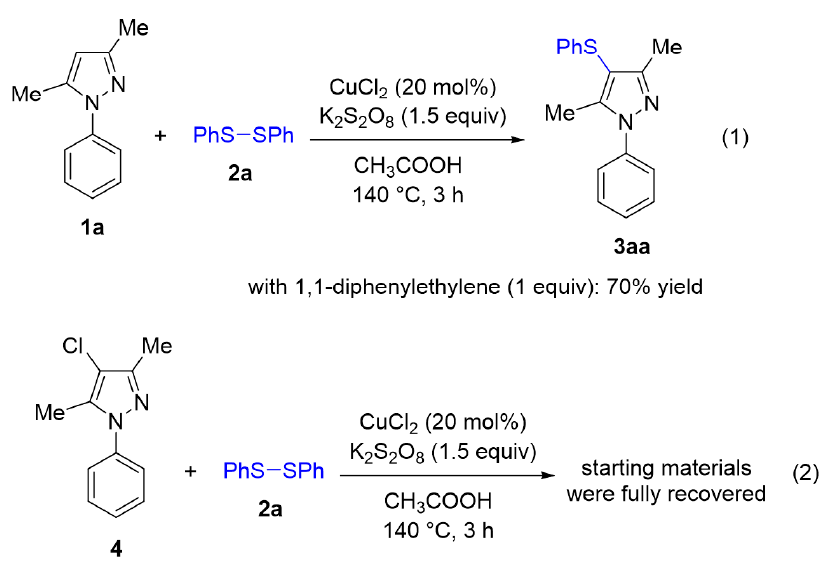
To understand the reaction mechanism, several control experiments were carried out (Scheme 3). The sulfenylation in the presence of 1,1-diphenylethylene as a common radical quencher still afforded 3aa in 70% yield (equation 1). We also did not observe the formation of any vinyl thioether adduct in the crude mixture, somewhat indicating that the reaction mechanism should not involve any radical species. Treating a 4-chloro-substituted pyrazole 4 under standard conditions did not give any product (equation 2). This result implied that the reaction mechanism was not simply a sequence of electrophilic chlorination/nucleophilic sulfenylation. Based on those results, a possible mechanism was proposed (Scheme 4). It should start with oxidation of CuCl with diphenyl disulfide 2a in the presence of KSO oxidant, thus yielding the Cu(III) species 4. An electrophilic substitution of 1a with 4 would selectively occur at C4−H bond of pyrazole due to the conjugation effect of N1 atom, thus furnishing the aryl-copper adduct 5. Reductive elimination in 5 would give the sulfenylated product 3aa and a Cu(I) species which was then oxidized in the presence of KSO to regenerate the active copper complex.
CONCLUSIONS
In conclusion, we have developed a method for direct sulfenylation of C4−H bonds in -aryl pyrazoles with diaryl disulfides. Reactions proceeded well in the presence of catalytic amounts of CuCl, KSO oxidant, and CHCOOH solvent. Seven different unsymmetric diaryl thioethers were isolated, varying from moderate to good yields. Functionalities, including bromo, chloro, and nitro groups, were tolerated under reaction conditions. A possible mechanism for selective sulfenylation was also rationalized, as electrophilic sulfenylation should be favored.
LIST OF ABBREVIATIONS
GC: gas chromatography
GS-MS: gas chromatography mass spectrometry
H NMR: proton nuclear magnetic resonance.
C NMR: carbon-13 nuclear magnetic resonance
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDEMENT
The Viet Nam National Foundation for Science and Technology Development (NAFOSTED) is acknowledged for supporting this research under project code 104.01-2019.354.
AUTHORS’ CONTRIBUTIONS
Nguyen Thanh Tung: Conceptualization, Writing - Original draft.
Pham Quoc Anh: Investigation, Formal analysis.
Pham Hoang Hai: Investigation, Formal analysis.
Le Vu Ha: Methodology, Validation.
All authors read and approved the final manuscript.

