Improving hairy root induction of Urena lobata L. by Agrobacterium rhizogenes ATCC 15834 by some factors
- University of Science, VNU-HCM, Ho Chi Minh City, Viet Nam
Abstract
Introduction: Our previous study showed that Urena lobata L. hairy root is a potential pharmaceutical source for type 2 diabetes treatment. In order to improve the transformation efficacy and the quality of hairy roots, this study examined the effects of several factors including age, parts of plants, infection time and culture medium in inducing hairy roots in Urena lobata L.
Methods: In this study, we investigated four factors to improve the hairy root induction in Urena lobata L. These factors include: age of plant (15-day-old in vitro plants, 45-day-old in vitro plants and after two subculture generations plants), different parts of plant (roots, stems, and leaves), infection time (10, 20 and 30 minutes), and culture medium (Murashige and Skoog (MS), Gamborg B5 medium (GB5) and Woody plant medium (WPM)). All experiments were repeated three times, with uninfected leaf explants of 15-day-old in vitro as the negative control. The transformation frequency and the fresh biomass of hairy roots were recorded at four weeks after infection.
Results: The results showed that the optimized procedure which used 15-day-old in vitro plants, the leafy part, the infection time of 10 minutes and culture in the WPM medium was better than the original procedure. The optimized procedure achieved a transformation frequency of 100%. In addition, the fresh biomass of hairy roots formed on an explant in the optimized procedure was 3.2 times higher than the ones induced by the original procedure.
Conclusion: The results showed that the optimized procedure was more effective than the original procedure in inducing Urena lobata hairy roots.
Introduction
L. belongs to the Malvaceae family, which is used in herbal medicine to treat a wide range of ailments such as colic, malaria, gonorrhea, fever, wounds, toothache, rheumatism 1, and especially diabetes 2. Studies have shown that the extracts from L. have medicinal activities such as antioxidant, anti-inflammatory, antimicrobial, antidiarrheal, antidiabetic, anti-hyperlipidemic, and anti-diarrhoeal activities 13. In addition, studies on phytochemical have analyzed and identified different compounds from L. extracts such as alkaloids, falconoids, tannin, saponin, coumarin, steroid/triterperioid, furocoumarin, mangiferin, quercetin, imperatorin, β-sitosterol, kaempferol, luteolin, hypolatin, gossypetin, and stigmasterol 456.
In plant tissue culture, hairy root culture technique is a key step in the production of secondary compounds . Hairy roots are generated by infecting T-DNA into the genome of the plant. The conditions of the gene transfer (the nature and age of the plants, the bacterial strain, the bacterial density, and the infection process) have a great influence on the frequency of gene transfer as well as the growth and yield of the hairy roots. For examples, hairy root induction in Rosmarinic acid content in transformed roots (213.42 μg/g dry wt) was significantly higher than non-transformed roots (52.28 μg/ g dry wt) 7. Moreover, hairy root induction in L., ATCC15834 strain and the excised seedling as explant produced the highest number of hairy roots 8. After optimizing culturing parameters (medium composition, , ), hairy roots can be optimized to grow rapidly and produce valuable compounds 9.
Due to the medicinal properties of L. and the advantage of hairy root culture technique, this study aimed to optimize hairy root induction in culture of L. to produce highly bioactive materials for the pharmaceutical industry. Therefore, this study focused on examining factors (age, plant parts, infection time, and culture medium) affecting hairy root induction to improve the hairy root induction and to increase the transformation frequency of L.
Methods
Chemicals
Taq polymerase, 100 bp Plus Ladder were purchased from Bioline. BC,G were produced by Integrated DNA Technologies.
Sterilization of culture materials
Seeds of L.was locally collected in district 9, Ho Chi Minh City, Vietnam. The selected seeds had good quality and free from infection. The seeds were washed with 80% ethanol for two minutes. Then, the seeds were shaken and soaked in 2% sodium hypochlorite for 10 minutes and then washed with sterile distilled water. Seeds after sterilization were placed on the MS (Murashige and Skoog) medium supplemented with 3% (w/v) sucrose and 0.8% (w/v) phytoagar (pH 5.8). The seeds germinated in a growth chamber at 25 ± 2C under standard cool white fluorescent tubes with a 16-h/8-h photoperiod. Plantlets were collected at different ages depending on the purpose of the experiment.
Investigating factors affecting hairy root induction in L.
Preparation of Agrobacterium rhizogenes
ATCC15834 strain was obtained from RIKEN bank (Japan) through the MEXT project. ATCC15834 cells were grown in a nutrient broth medium (beef extract 3 g/L, peptone 5 g/L, pH 7.0) for 48 hours in a shaking incubator (110 rpm, 25 ± 1C).
The original procedure for hairy root induction
The original procedure used for L. hairy root induction was as following 10: the leaves from 15-day-old plants were injured on the surface to facilitate the infection process. These segments were soaked in the ATCC15834 suspension (OD = 0.6) for 20 minutes. After four days of co-cultivation, the explants were transferred to the MS medium (3% sucrose) supplemented with cefotaxime (250 mg/L) to eliminate the remained ATCC15834. The samples were grown under dark conditions at 25C for hairy root induction. Within two weeks, numerous hairy roots emerged from the wounded sites on leaf explants. The number of responsive explants and number of hairy roots per explant were recorded 30 days after infection.
Besides the investigated factors, the remaining factors were similar to the original hairy root induction procedure. All experiments were repeated three times, with the same negative control is leaf explants from 15-day-old seedlings that were not infected with .
The effect of age and different parts of plantson hairy root induction
Roots, stems, leaves of three types of plants: 15-day-old plants, 45-day-old plants and after two subculture generations plants were infected with to induce hairy roots.
The effect of infection time on hairy root induction
Samples were infected with for 10 minutes, 20 minutes, and 30 minutes to determine the optimal infection time.
The effect of culture medium on hairy root induction
Three types of medium were used: MS, GB5 (Gamborg B5 medium) and WPM to determine the optimal culture medium.
The effect of the combined of improved factors on hairy root induction
The hairy roots were induced in two procedures: the original procedure and the optimized procedure with optimized conditions (age, plant parts, time of infection and medium of induction).
Confirmation of transgenic roots
Genomic DNA samples were extracted from hairy roots and roots (non-transgenic roots) by the CTAB method as described previously 11. The Ri-plasmid was isolated from A. rhizogenes ATCC 15834 by the method described by Curier and Nester 12. PCR reactions were performed using the genomic DNA from the hairy roots and non-transgenic roots as well as the Ri-plasmid with specific primer sets for rolB, rolC, and virG genes. The sequencing primers include F-rolB (5’- GCTCTTGCAGTGCTAGATTT-3’), R-rolB (5’-GAAGGTGCAAGCTACCTCTC-3’); F-rolC (5′-CTCCTGACATCAAACTCGTC-3’), R-rolC (5’-TGCTTCGAGTTATGGGTACA-3’); and F-virG (5’-TTATCTGAGTGAAGTCGTCTCAGG-3’), R-virG (5’-CGTCGCCTGAGATTAAGTGTC-3’).
The expected amplified fragment sizes were 423 bp for gene, 626 bp for C gene, 1030 bp for G gene 13. The PCR reactions were performed in a total volume of 25μl containing 100ng of plant genomic DNA (or 40 ng of Ri-plasmid DNA), 5 µL Taq polymerase buffer (5X), 0.5 µM of each primer and 1U Taq polymerase (Bioline). PCR steps included initial denaturation at 95C for 5 minutes, followed by 35 cycles of amplification (95C for 30s, 54C for 30s and 72C for 60s) and a final extension at 72C for 10 minutes. PCR products were visualized by agarose gel electrophoresis.
Statistical analysis
Each treatment included 20 explants in replicates of three. The transformation frequency was calculated at four weeks after infection. All data analyses were performed using the SPSS 16.0 (Copyright SPSS Inc.). Experimental results were shown as mean ± standard deviation (SD). Differences between means were evaluated by Duncan’s multiple range tests. Statistical significance was accepted at 0.05.
Results
Sterilization of culture materials
The seeds of L. were sterilized by 2% sodium hypochlorite in 10 minutes. The germination rate was 90-100%. After two days on MS medium, germinated seeds developed into seedlings. 45-day-old plants were mature enough and suitable for subculture. Seedlings were collected at different ages depending on the specific purposes of each experiment (Figure 1).

Investigating important factors to improve the hairy root induction in
The effect of age and different parts of plants on hairy root induction
After four weeks after infection, L. leaves had the highest transformation frequency compared to the stems and roots at all ages (
The effects of age and parts of plants on transformation frequency four weeks after infection (%)
| Parts of | Transformation frequency (%) | |
| After two subculture generations plants | Root | 7.333f ± 2.081 |
| Stem | 6.333f ± 1.528 | |
| Leave | 23.000e ± 2.645 | |
| 45-day-old | Root | 8.333f ± 2.081 |
| Stem | 32.667d ± 3.055 | |
| Leave | 40.000c ± 3.000 | |
| 15-day-old | Root | 30.333d ± 2.517 |
| Stem | 86.333b ± 2.517 | |
| Leave | 97.333a ± 2.082 |
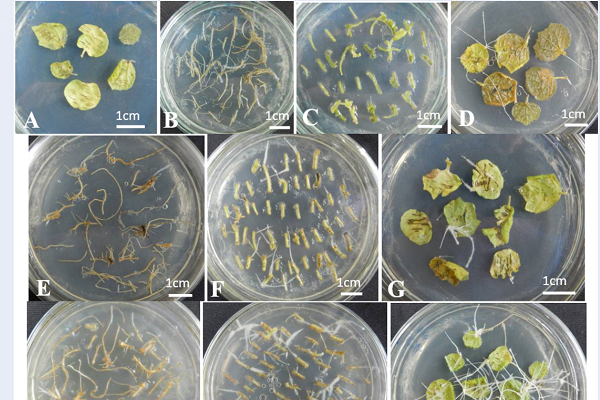
The effect of age and parts of plants on hairy root induction four weeks after infection. (A) uninfected leaf explants (negative control). (B, C, D) explants form roots, stems, leaves of after two subculture generations, (E, F, G) explants form roots, stems, leaves of 45-day-old
The effect of infection time on hairy root induction
The results indicated that the infection time of 10 and 20 minutes had a similar transformation frequency (97.67% and 97.33%, respectively) (
The effect of infection time on hairy root induction after four weeks of infection
| Infection time (minute) | Transformation frequency (%) |
| 10 | 97.667a ± 2.517 |
| 20 | 97.333a ± 2.082 |
| 30 | 93.667b ± 3.215 |

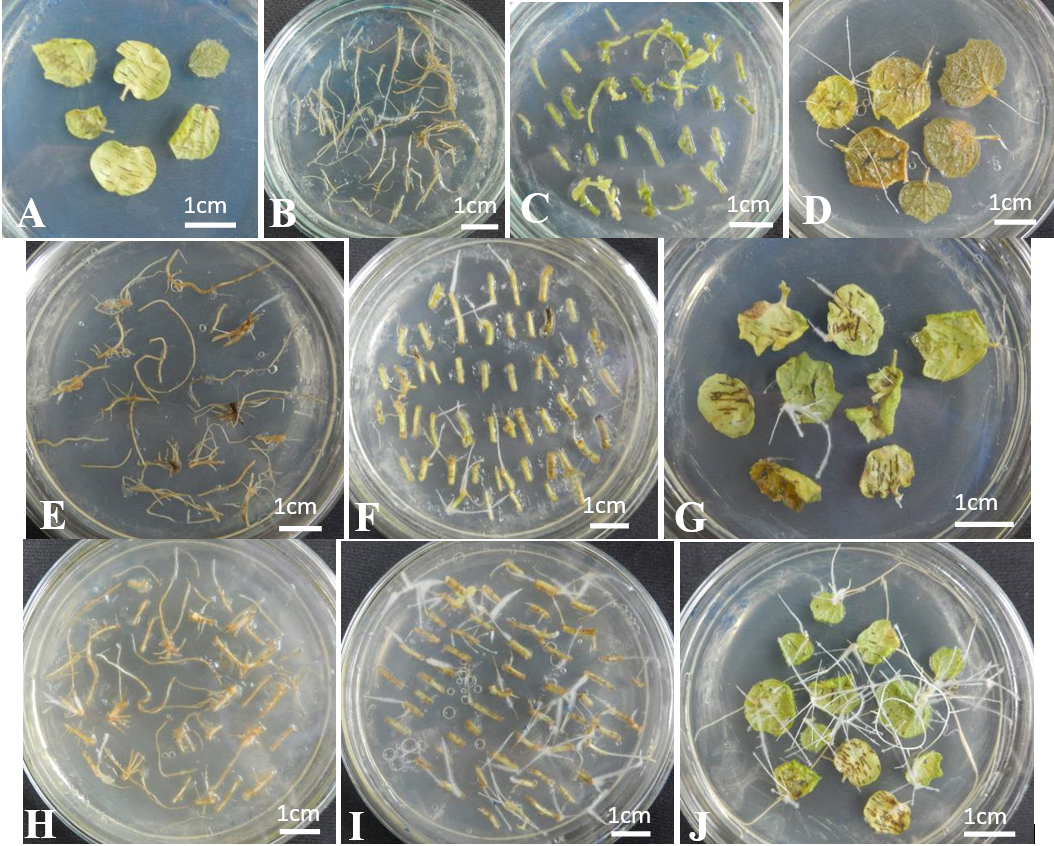
The effect of infection time on hairy root induction four weeks after infection. (A) uninfected leaf explants (negative control), (B, C, D) infected with
Effect of culture medium on hairy root induction
The results showed that WPM and MS medium had a similar transformation frequency four weeks after infection (100% and 97.3%, respectively). GB5 medium had the lowest transformation frequency (91.67%) four weeks after infection (
The effect of culture medium on hairy root induction four weeks after infection
| Culture media | Transformation frequency (%) |
| MS | 97.333a ± 2.082 |
| WPM | 100.000a ± 0.000 |
| GB5 | 91.667b ± 1.528 |

The effect of culture mediums on hairy root induction after three weeks after infection. (A) uninfected leaf explants (negative control), (B, C, D) explants infected with
The effect of the optimized conditions on hairy root induction
After identifying the optimal conditions for the hairy root induction in L., these conditions were combined and compared to the original procedure. Specifically, 15-day-old leaves were infected for 10 minutes and cultured in WPM medium. The results in the table 4 and the figure 5 showed that the optimized conditions had the transformation frequency of 100%, while the original procedure achieved a transformation frequency of 97.33% (
The combined effect of the optimized condition on hairy root induction four weeks after infection
| Induced process | Transformation frequency (%) | Fresh weigh/explant (g) |
| Original condition | 97.333 ± 2.082 | 0.160 ± 0.053 |
| Improved condition | 100.000 ± 0.00 | 0.517 ± 0.076 |

The combined effect of improved factors on hairy root induction after four weeks of infection. (A) uninfected leaf explants (negative control). (B) Hairy roots are induced by the original process. (C) Hairy roots are induced by the optimized process.
Transgenic roots were confirmed by PCR
The hairy root samples were analyzed by PCR to test whether the transgenic process was successful. DNA samples from the putative hairy roots and from non-transgenic roots were isolated and subjected to PCR analysis for the presence of ,, and genes, which are present in Ri-plasmid. The -plasmid of ATCC15834 was also included to serve as a positive control. The presence of and, as well as the absence of virG from these hairy roots confirmed that -plasmid was integrated successfully into the plant genome (Figure 6).
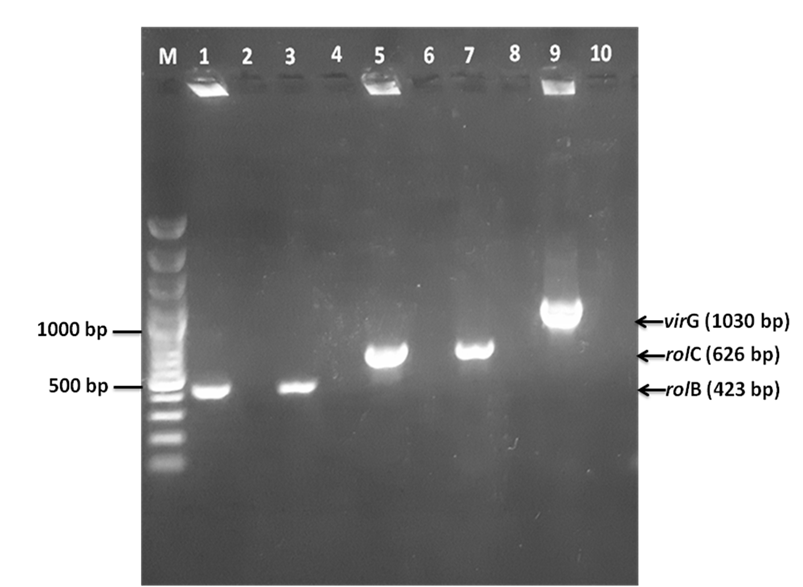
Confirmation of transformation by PCR. PCR amplification of
Discussion
The age and parts of plants are important factors influencing the transformation of into plants. Young plants and seedlings have higher transformation efficacy than tissues and organs from mature plants. Young plant tissues such as hypocotyl, cotyledon, and young leaves are often used for infection to induce hairy roots 14. Our data in L. also showed that leaves from 15-day-old plants have the highest rate of hairy root induction. In 15-day-old L., the leaves are cotyledons, and the stem is hypocotyl. Hence, they are good materials for infection. In addition, many studies also showed that leaves have the highest rate of hairy root induction. This observation was attributed to the ability of leaves to produce a large number of cells in wound healing response. Wound healing response is the most important factor in inducing hairy roots.
The optimal infection time to induce hairy root for each plant species is different. For example, the optimal infection time to induce hairy root in L. is 10 minutes. Likewise, the optimal infection time for DC is 3 hours 15 and L. is 20 minutes 16. Therefore, it is necessary to optimize the infection time for each plant species to achieve the highest hairy root induction.
The culture medium is one of the most important factors influencing the induction as well as the growth of hairy roots. In this experiment, WPM medium was the optimal media to support the induction and growth of hairy roots in L., which indicates that the composition and the mineral content in WPM medium were suitable for the growth of the L. hairy roots. WPM medium is frequently used to support the growth of woody species, which may support the growth of a subshrub plant like L. better than MS and GB5 medium.
Conclusions
This study identified important factors that can improve the hairy root induction in L. by (ATCC 15834). These factors include parts of plants, age of plants, infection time and culture medium. By using the optimized conditions (leaves of 15-day-old plants, infection time of 10 minutes, WPM medium), 100% transformation efficacy was achieved, and the fresh weight of hairy roots per explant was 3.2 times higher than that of the original procedure.
Competing Interests
The authors declare that they have no conflict of interest.
Authors' Contributions
Vu Thi Bach Phuong implemented the experiment and wrote the manuscript. Quach Ngo Diem Phuong proposed ideas and reviewed. Pham Thi Anh Hong is the advisor.
Acknowledgments
This research is funded by Vietnam National University HoChiMinh City (VNU-HCM) under grant number C2018-18-18.

