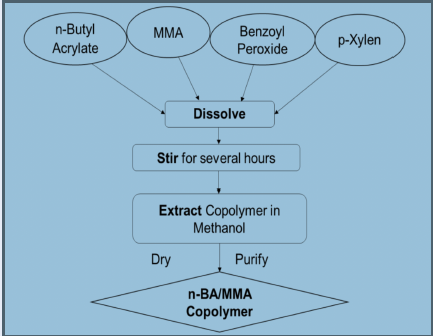
Copolymerization of n-butyl acrylate/methyl methacrylate in xylene solvent
- 1. Faculty of Materials Technology, Ho Chi Minh City University of Technology, VNU-HCM
- 2. National Key Laboratory of Polymer and Composite Materials, Ho Chi Minh City University of Technology, VNU-HCM
- Nguyen Chi Thanh High School, Khanh Hoa Province, Viet Nam
Abstract
Introduction: Copolymers are being used in a variety of fields because of their diversity and since any change could result in significant modifications. The butyl acrylate/methyl methacrylate copolymer is an extensively used polymer system with many advantages. This study aims to analyze some changes in copolymerization, from using different time or temperature of synthesis, in order to find an optimal process that can be applied in Vietnam.
Methods: Copolymerization of butyl acrylate/methyl methacrylate copolymer with different butyl acrylate/methyl methacrylate monomer ratios was done using Xylene solvent. Copolymer compositions were determined from the Proton nuclear magnetic resonance spectra and Gel permeation chromatography.
Results: The experimental results indicate that the copolymer was successfully synthesized. The highest molecular weight was 15,591 g/mol, with the lowest dispersity of 1.53 at 100 oC after 6 hours of copolymerization.
Introduction
The commercial Acrylic resins are usually copolymerized between acrylate monomer and methacrylate monomer together or with styrene or other monomers, due to economic and/or technical reasons. The nature and the components of the Acrylic resin have a significant influence on weather resistance and other properties of Acrylic plastic-like transparent, hardness, and flexible pigment combinations. Poly (methyl methacrylate) (PMMA) is so stiff that the resin has not responded to products which require high impact strength as well as bending strength, such as domes and shoe soles. Moreover, the poly (n-butyl acrylate) (PBA) is flexible and easy to bend 1. The copolymerization of BA and MMA creates a new resin which can overcome the weaknesses of each polymer and enhance the physical properties. The butyl acrylate/methyl methacrylate (BA/MMA) copolymer has some properties of both PBA and PMMA resin, including being transparent, easy to combine with pigments, able to be bent, and having high adhesion. This copolymer can be applied in exterior decorations and domes.
Since 1981, the BA/MMA copolymers have been evaluated in copolymerization methods, such as free radical synthesis of polymers with hydroxylated ends 2,3,4, bulk copolymerization of this system 5, and seeded semi-batch emulsion copolymerization 6. The n-BA/MMA copolymer has shown many beneficial prospective characteristics, such as weather tolerance, high transparency, and high adhesion. Due to these features, the n-BA/MMA copolymer has been extensively studied for applications in painting, adhesive, and coating. Recently, an article reported on the kinetic free-radical copolymerization of n-butyl acrylate with methyl methacrylate in solution 3, the reactivity ratios on the conventional copolymerization 7, and the effect of solvent on the rate constants in solution polymerization 8,9. However, some factors (such as reaction time, temperature, and solvent types) which affect the system have not been studied properly yet. Thus, the goal of this research study is to specifically investigate the factors mentioned above.
In Vietnam, the BA/MMA copolymer is imported to meet the demand, although the application is vast including factory floor coating, finishes, polishes, paints, and adhesives. For the above, there has not been any local manufacturers as of yet. The BA/MMA copolymer is an Acrylic resin that has precious properties, which include strong resistance to weather conditions in Vietnam. Thus, the successful research and synthesis of this copolymer would contribute a new material for the domestic material industry, helping Vietnam become a proactive leading synthesizer of this polymer.
This research study also aims to develop a suitable process for the climate in Vietnam and to study a variety of emulsion polymerization as well as different catalysts, solvents, and conditions of synthesis ( temperature and time, .). In further research, it is recommending examining the ability to combine with different additives to find more advantages, increase good properties like adhesion of this copolymer and reducing its weakness.
Experiments
Materials
Monomer n-butyl acrylate was purchased from Sinopec (China), and monomer methyl methacrylate was obtained from Vinachem (Vietnam). Benzoyl peroxide was purchased from Merck (Germany) and was used as an initiator. P-xylene, and methanol were obtained from Vinachem, and were used as solvents in copolymerization reactions.
Synthesis of n-BA/MMA copolymers
MMA monomer and n-BA monomer were mixed in p-xylene solvent at a concentration of 1.5 mol/L for each monomer in a sealed glass flask fitted with a reflux condenser. After that, benzoyl peroxide at 5x10 mol/L concentration was added into the reaction system as an initiator. The evaluated temperature conditions were 80 °C, 90 °C, and 100 °C for the desired time (at least 2 hours). During the process, the reaction mixture was stirred frequently. After the desired time, the entire volume was poured into a large excess of methanol in a vessel. The copolymer was purified by repeated precipitation in methanol solvent. The precipitated polymer was purified then filtered and dried in a vacuum until the constant weight was reached (Figure 1).

Synthesis of n-BA/MMA copolymers in p-xylene.

Copolymerization reaction of Butylacrylate and Methyl methacrylate.
The reaction of n-BA/MMA copolymerization was conducted following Scheme 1; the MMA unit and n-BA unit were dependent on the feeding ratio of n-BA and MMA monomers.
Proton nuclear magnetic resonance (H-NMR)
The H-NMR spectrum was investigated using a Bruker Advance machine (Vietnam Academy of Science and Technology, Hanoi, Vietnam) at 500 MHz and was used to determine the molecular structures of the n-BA/MMA copolymer. Their spectra were analyzed in deuterochloroform (CDCl) solvent, and tetramethylsilane (TMS) was used as an internal reference. The mole fraction of MMA unit and n-BA unit in the copolymer was calculated from the H-NMR spectra using the following equation 10,11:
I(OCH) is the area under the resonance signal of (OCH) protons of the MMA unit.
I(OCH) is the area under the resonance signal of (OCH) protons of the n-BA unit.
Fis the mole fraction of MMA unit in the copolymer.
Fis the mole fraction of n-BA unit in the copolymer.
Gel permeation chromatography (GPC)
The molecular weight (Mn) and Polydispersity index (PDI) of n-BA/MMA copolymers were measured by gel permeation chromatography (GPC) PL-50 Plus (Agilent Technology, USA) with a PLgel mixed column (obtained from the National Key Laboratory of Polymer and Composite Materials, Vietnam National University, Ho Chi Minh City (VNU-HCM)) at room temperature. Chloroform was used as an eluent at a flow rate of 1 mL/min. Calibration was carried out using polystyrene standards of Polymer Laboratories Inc.
Results
Characteristics of the n-BA/MMA copolymer using H-NMR
The H-NMR spectrograph confirmed that the n-BA/MMA copolymer was synthesized successfully. The highest peak at 0.93 ppm (6) represents the hydrogen of methyl (-CH) groups of the n-BA unit. The methyl hydrogen (-CH) groups of (-(CH)C(CH)-COO-) and (-COO-CH-) linkages of the MMA unit were observed from 0.9 to 1.12 ppm (b) and 3.6 ppm (a), respectively. The signals at 1.60 ppm (4), 1.38 ppm (5), and 1.89 ppm (1) were assigned to methylene hydrogen (-CH-R) of the n-BA unit. The methylene hydrogen (-CH -) group (peak c) of MMA unit was observed at 2.3 ppm. The shift δ=4.04 ppm (3) represented the specialized methylene hydrogen (-CH -) in ester linkages (-COOCH-) of the n-BA unit. The particular shifts on the H-NMR spectrum were according to the de-shield effect and H-NMR table 10,12.
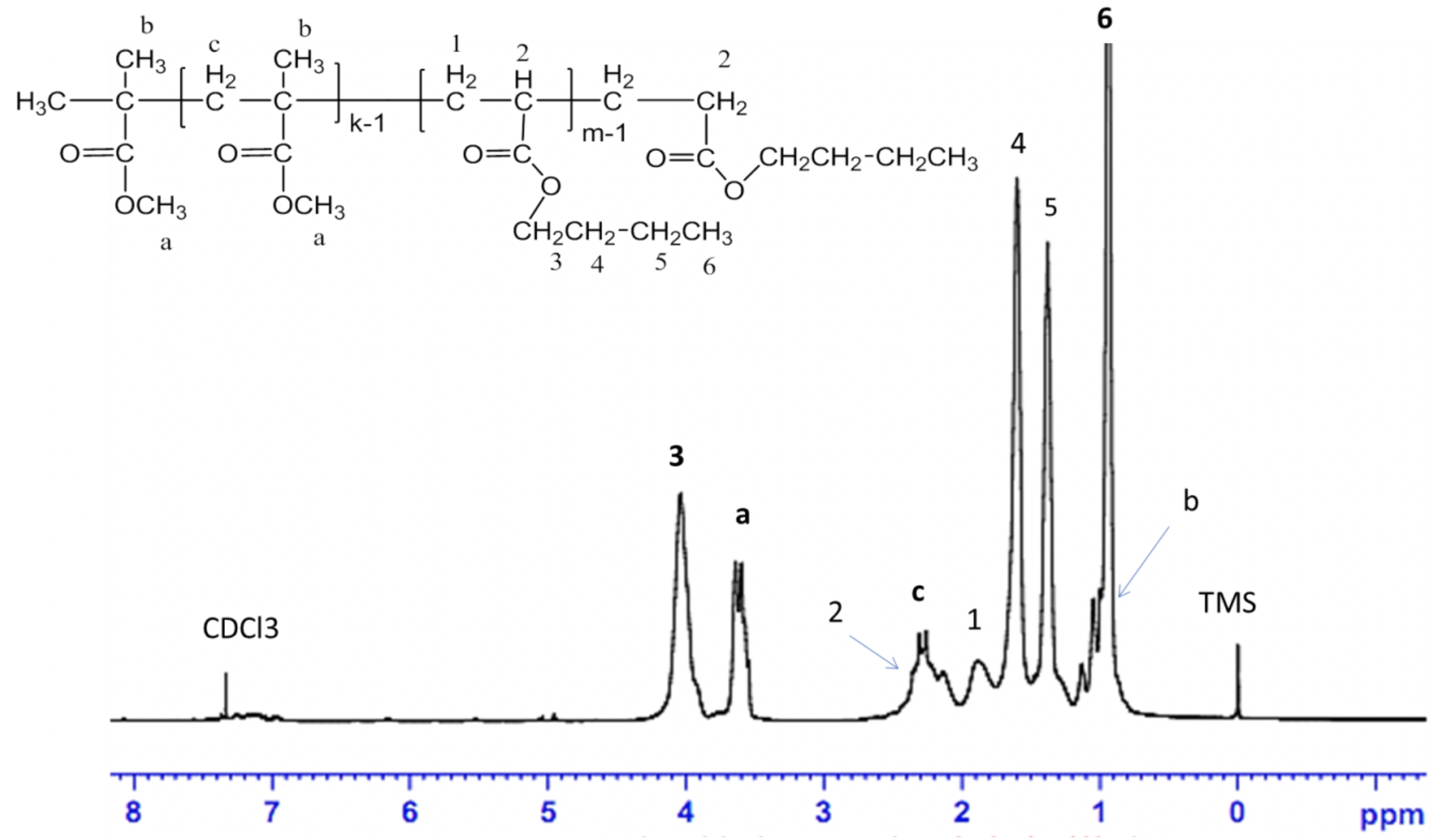
1H NMR spectrum of n-BA/MMA copolymer in CDCl3 (n-BA/MMA ratio=75/25, reaction temperature 100 oC, 6 hours).
The peak (6) and peak (3) on H NMR spectrum specify the n-BA unit, while the peak (a) and peak (c) specify the MMA unit in the n-BA/MMA copolymer structure. Modification of the n-BA/MMA monomer ratio led to changes in the height of peaks (a) and (c), and peaks (3) and (6).
Effects of temperature on the productivity of the synthesis reaction
At the low temperature of 80 C, the reaction nearly did not happen; as a result, the reaction productivity was 0.21 %. By increasing the reaction temperature, the polymerization was obtained and was insignificant at 90 C (the reaction productivity was 18.3%). While it happened effectively at 100 C (the reaction productivity was 56%) (Figure 4). The reaction productivity increased when the temperature increased and the optimal reaction temperature is 100C in these experiments.

Effect of temperature on reaction productivity of n-BA/MMA copolymerization in 6 hours (n-BA/MMA ratio=50/50).
Effect of reaction time on the productivity of copolymerization
Figure 5indicates that the n-BA/MMA copolymerization happened at 100 C and the productivity of copolymer increased gradually from 15.57 % to 55.49 %, when reaction time increased from 1 hour to 6 hours. Besides, the reaction productivity of n-BA/MMA copolymerization was obtained as 55.02 % (after reaction time of 5 hours) and was 55.4 % (after reaction time of 6 hours).
These results demonstrate that the extent of copolymer increase since the copolymerization happened were dependent completely on synthesis parameters (such as time reaction of 6 hours, 100 C temperature, and a mole ratio of Benzoyl Peroxide/(BA+MMA) of 0.00025/0.15 in xylene. The n-BA/MMA copolymerization in xylene solvent using Benzoyl Peroxide initiator was optimal at 100 °C with a reaction time of 6 hours.

Effect of reaction time on reaction productivity of n-BA and MMA copolymerization at 100 oC (n-BA/MMA ratio=50/50).
Effect of time and temperature on molecular weight (Mn) and Polydispersity index (PDI) of n-BA/MMA copolymer
At 100 °C, the molecular weight (Mn) of the copolymers was obtained from 9,915 g/mol to 15,591 g/mol and they were proportional to the reaction time of 3, 4, 5 and 6 hours, while PDI of copolymers was decreased from 2.08 to 1.53. However, Mn and PDI of n-BA/MMA copolymers changed when the time reaction decreased at 90 C (
Mn and Polydispersity of n-BA/MMA copolymer for the different reaction times and reaction temperatures (n-BA/MMA ratio=50/50)
| Temperature (oC) | Reaction time (hours) | Mn (g/mol) | PDI = Mw/Mn |
| 90 oC | 3 | 11,499 | 1.84 |
| 4 | 11,751 | 1.80 | |
| 5 | 11,974 | 1.79 | |
| 6 | 12,035 | 1.76 | |
| 100 oC | 3 | 10,808 | 2.08 |
| 4 | 9,915 | 2.12 | |
| 5 | 13,932 | 1.55 | |
| 6 | 15,591 | 1.53 |
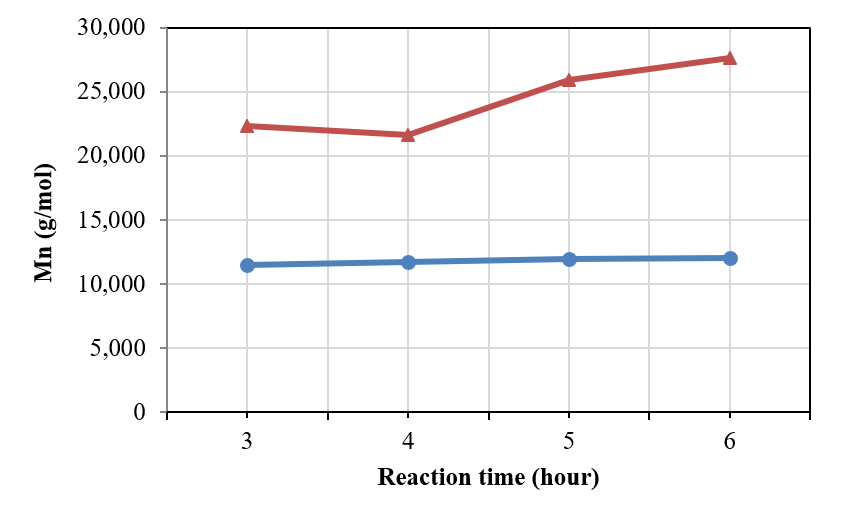
Effects of reaction time on molecular weight (Mn) of the n-BA/MMA copolymer (line with circle marker at 90 oC and line with triangle marker at 100 oC) (n-BA/MMA ratio=50/50).
As seen in Figure 6, the molecular weight of the n-BA/MMA copolymer increased gradually when the reaction time increased — from 3, 4 and 5 hours, to 6 hours. At 100 °C, the copolymer was obtained at the greatest molecular weight (15,591 g/mol) after 6 hours of reaction. In addition, the Mn of copolymer was nearly unchanged as the reaction time increased to 90 °C. The reason is that copolymerization happened significantly at 100 C such that increasing the reaction time caused the increase of Mn.

Effects of reaction time on Polydispersity index (PDI) of the n-BA/MMA copolymer (line with circle marker represents 90 oC and line with triangle marker represents 100 oC) (n-BA/MMA ratio=50/50).
Similar with Mn, PDI of copolymer was approximate when the reaction temperature was 90 C, even though the reaction time increased from 3 hours to 6 hours. At 100 C, the PDI of the copolymer decreased from 2.09 to 1.53, as the reaction time increased from 3 hours to 6 hours (Figure 7). If in 3-4 hours the copolymerization does not happen completely, it could be that PDI of the copolymer is high (2.09 and 2.12). However, the PDI of the polymer was obtained as 1.53 by increasing the reaction time to 6 hours since the reaction continues to happen with all monomers which do not react.
Effect of mole ratio n-BA/MMA on polymer structure
I(OCH) is the area under the resonance signal of (OCH) protons of the MMA unit.
I(OCH) is the area under the resonance signal of (OCH) protons of the n-BA unit.
Fis the mole fraction of MMA unit in the copolymer;
Fis the mole fraction of n-BA unit in the copolymer.
The mole fraction of the n-BA unit and MMA unit in the copolymer structure with different n-BA/MMA monomer ratios (reaction at100 oC, 6 hours)
| Mole ratio of n-BA/MMA | 25/75 | 40/60 | 50/50 | 75/25 |
| I(OCH2) | 22.64 | 44.90 | 194.78 | 2.00 |
| I(OCH3) | 120.18 | 147.35 | 444.68 | 1.16 |
| FBA (%) | 22.03 | 31.37 | 39.65 | 72.12 |
| FMMA (%) | 77.97 | 68.63 | 60.35 | 27.88 |
Discussion
The temperature affected the reaction productivity; at low temperature (e.g. 80 C), the copolymerization did not occur due to lack of energy for the n-BA and MMA reactions. At temperatures below 80 C, the n-BA monomer did not react with the MMA monomer, even when the reaction time was increased to 6 hours. Furthermore, at temperatures above 100 C, the copolymerization was unstable due to evaporation of the MMA monomer (T = 101 C). Therefore, the temperatures of 90 C and 100 C were chosen and used to evaluate the effects of reaction time on productivity, the molecular weight, and the polydispersity index of the n-BA/MMA copolymer. When reaction time was increased, the n-BA and MMA monomers have enough time to meet and react such that the productivity and the molecular weight of the copolymer increased. However, the n-BA and MMA monomers need more heat energy to form the n-BA/MMA copolymer. That is why the copolymerization consequently happened incompletely at 90 C. The molecular weight of the copolymers were approximately similar at the different reaction times from 3 to 6 hours. The n-BA/MMA copolymerization happened significantly at 100 C such that increasing the reaction time caused an increase of molecular weight.
The I(OCH) and I(OCH) were calculated based on the H-NMR spectra; the copolymers were synthesized successfully with all n-BA/MMA monomer ratios. The structure of the copolymer could be controlled by modifying the n-BA and MMA monomer mole ratios. Depending on the demand of copolymer properties and their applications, the structure as well as the composition of PBA and PMMA in the n-BA/MMA copolymer backbone can be designed.
Conclusions
Copolymerization of n-BA and MMA occurred at temperatures over 90 C using Benzoyl peroxide initiator and p-xylene solvent. However, the productivity of the reaction was the greatest at the temperature of 100 C and reaction time of 6 hours. At 90 C, the molecular weight and polydispersity index of the n-BA/MMA copolymer were mostly unchanged by any increases in reaction time. At 100 C, the molecular weight of the copolymer was increased and polydispersity index was reduced gradually when reaction time increased from 3 hours to 6 hours.
The product was the n-BA/MMA copolymer with random structure in which the mole fraction of the n-BA unit was directly proportional to the fraction of n-BA monomer which reacted in the copolymerization.
Abbreviations
H- NMR : Proton nuclear magnetic resonance
CDCl : Deuterochloroform
GPC : gel permeation chromatography
PBA : Poly (n-butyl acrylate)
PMMA : poly (methylmetacrylate)
PDI : Polydispersity index
MMA : methylmetacrylate
Mn : The molecular weight
n-BA : n-buthyl acrylate
TMS : tetramethylsilane
Competing Interests
The authors declare no conflict of interest related to this research.
Authors' Contributions
Nguyen V. V. Linh wrote the paper and was responsible for GPC results and H NMR spectra. Nguyen. T. Duc synthesis the n-BA / MMA copolymer and evaluated the reaction productivity. Huynh D. Phu was the corresponding author and responsible for the revision and content of this study. All authors provided data analysis and read and approved the manuscript.

