
Serological and Molecular Techniques for the Diagnostic of Brucellosis
- College of Life Science, Northwest University, Xi’an, Shaanxi, P.R China 710069.
- Department of Microbiology, Hazara University, Mansehra, Khyber Pakhtunkhwa, Pakistan
- College of life science, Northwest University, Xi’an, Shaanxi, China, 710069.
- College of biotechnology, Tianjin University of Science and Technology, Tianjin 300457.
- Center for Emerging Infectious Diseases, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan 430071, China.
Abstract
Brucellosis is known as undulant fever or Malta fever, caused by the genus Brucella. It is the most common human zoonosis. The disease is worldwide distributed and causes significant economic losses. In animals, it causes abortion, reduction in milk production, and infertility. While brucellosis in humans is a debilitating disease with various clinical manifestations that may lead to death in some cases. Control of disease in animals needs proper diagnosis, permanent monitoring of brucellosis-free herds, and removal of infected animals. The current review will discuss the serological and molecular techniques daily used for the determination of brucellosis in animals and humans.
Introduction
Human is a significant zoonosis with a worldwide geographical distribution. The causative agents of belong to the genus Brucella. The traditional human's disease generally caused by , , and . mostly transmitted to humans through direct contact with infected animal secretions, placentas, or aborted fetuses and by the consumption of unpasteurized milk and milk products. In cattle, causes reduce fertility, stillbirth, late birth, and reduced milk production resulting in significant economic losses. While in humans, its clinical manifestations are non-specific such as undulant fever, insomnia, malaise, nervousness, repression, and sexual impotence. in humans is also known for various organ involvement, causing meningitis, encephalitis, endocarditis, orchitis, arthritis, and prostatitis. Additionally, in pregnant women, causes spontaneous abortions1.
It is challenging to diagnose because signs and symptoms are almost similar to other infections; the causative agent usually grows very slowly in blood culture, and also the serodiagnosis is complicated2. can be diagnosed by using several serological tests using antibodies, but the gold standard remains isolation and identification of the bacterium. Cultural observations of Brucella are time consumable, non-sensitive, and hazardous to lab staff. Various attempts were made to diagnose for more than one century. Brucella diagnosed by using a combination of tests to avoid false-negative results3.
Therefore, this study aims to review diagnostic techniques used for the isolation, screening, epidemiological surveillance, and confirmatory for in humans and livestock.
Direct smear microscopic examination
The microorganism can be identified by microscopic examination of stained smear from secretions, fetuses, and exudates like vaginal discharges, placenta, using modified Ziehl-Neelsen (ZN) staining. This can provide a predictive diagnosis of , especially with serological support. Brucellae are not a true acid-fast bacillus but show resistant to decolorization by week acids. They seem like short rods or coccobacilli, mostly arranged singly but occasionally in pairs or small groups. They appear as coccobacilli or short rods, usually arranged individually but sometimes in pairs or small groups. Organisms such as and can resemble . The diagnoses of Brucella can sometimes be misleading by , , and because these bacterial strains are superficially similar to Brucella strains4. To identify and isolate accurately it is best to used vaginal swab and milk samples of goats and sheep and culture these samples on culture media Farrell,s selective media5.
Cultural isolation of organism
Brucella may be isolated from the placenta, fetus, vaginal swab, colostrum, milk, semen, the secretion of nonlactating udders, the testis and the sites of clinical localization such as hygroma fluids or infected joints. While the microscopy samples include various lymph nodes, spleen, the pregnant or premature post parturient uterus, the udder, and male reproductive organs6. At the research site, mostly culturing tests are used to diagnose . Culturing of Brucella from blood is useful in the case of bacteremia, which does not always exist but culturing milk gives a positive response to show the presence of Brucella. Samples of liver, udder, lymph nodes, spleen, and other organs used for culturing the purpose of . Phenotypic characters including CO requirement, phage typing, and biochemical tests, are of great deals while using culture techniques for the identification of Brucella organisms and other problems in culturing are time-consuming, trained interne and applications of bio-safety7. To culture brucella, broth or agar can prepare from powder media. Solid media, including tryptose agar, trypticase soy agar, and dextrose agar are used to identify and isolate Brucella at the primary level. However, species like and can be cultured in media by the addition of 5-10 % sterile bovine or equine serum to it8. The optimum pH for the growth of Brucella is 6.6-7.4, whereas, optimum growth temperature ranges from 36 to 38 °C9. Growth of other microbes and contaminants can be prevented using selective media such as Kuzdas and Morse and Farrell,s Morse10, 5.
Farrell’s medium has some drawbacks because some of Brucella strains such as and cannot show healthy growth. Therefore, Thayer- Martin medium is slightly modified and then used in combination with Farrell’s medium to get better growth of these Brucella species2.
Molecular methods
The molecular procedure often based on PCR amplification is dominantly used for identification and typing to reduce the problem and hurdles of microbiological testing11. DNA isolation is an initial and essential step of PCR as its feature has a considerable impact on method sensitivity12. Initially, for bacterial determination, PCR has been developed13. Also, now, these processes are applied for the identification of in humans and animals’ clinical samples. The use of a single pair of primer act to the bacterial DNA sequence, such as 16 S-23s RNA operon, 15711 or BCSP31 genes with PCR is a reliable technique for the detection of 14. Using a mixture of some primer’s pairs for magnification of BCSP31, OMP2B, OMP31 genes, encoding the external membrane proteins. It is easy to detect the four Brucella species: and. The mixture of seven PCR reactions is another to allocate favoritism between brucella six species. PCR techniques used for the detection of some Brucella abortus biovars, which differentiated between S19 and RB51 strain of and allowed for vaccination against pathogenic strain15.
Multiplex PCR
To boost the affective prevention and of , a quick and precise method is required. Several studies have developed a PCR based assay for the differentiation of Brucella species. It has been revealed that the two multiplex PCR, called AMOS (, , and ) and Bruce-ladder PCR assay can discriminate most of Brucella species such as marine mammal and vaccine strain RB51, S19 and 16. It allowed identification evidence of the four of Brucella species ( and ) and was titled AMOS PCR for the main correspondence of species name. AMOS PCR cannot detect the similar species single biovar but identify just the pair biovars of each of the same species, sooner after this technique has been promoted to differentiate more biovar and recognize brucella S 19 RB51 vaccine strain17, 16. Moreover, as the PCR system convey high contamination risk and needs equipment for visualization, it is less favorable for routine diagnosis purpose. So real-time PCR systems have been established that are quicker and less prone to contamination and thus use more clinically.
Real-time PCR
The real-time PCR method is highly specific, sensitive, reproducible and quicker than the conventional PCR. The quantitative real-time (qRT) PCR permits both identification and quantification of the PCR product in real-time, but it is synthesized18. It has also been possible to differentiate the species and even at the biovar level through real-time PCR. This technique can be used for the quick diagnosis of chronic serologically positive and for acute when blood and serum samples of recognized clinical presentations are examined19. These assays are developed for targeting 16 S-23 S internal transcribed spacer region (ITC) and the genes coding omp25 and omp31, bcsp31, and IS71120. For the detection of bacteria at the genus level, the bcsp31 gene target can be suggested. Species-specific recognition verifying the initial diagnosis by second gene target such as IS71121 (
Serological diagnosis
Several serodiagnosis methods are found for the determination of 26. However, some of the tests are satisfactory sensitive and specific like indirect enzyme-linked immunosorbent assay (i-ELISA), competitive enzyme-linked immunosorbent assay (c- ELISA), Milk ring test, complement fixation test (CFT) and the fluorescence polarization assay (FPA)27, 26. In each and every epidemiological situation, no single serological test is sufficient, all of which have limitations, particularly when it comes to screening individual animals Fluorescence polarization assay (FPA), Complement fixation test (CFT) and ELISA are considered more suitable for international trade than serum tube agglutination test (SAT). The buffered Brucella antigen tests (BBATs), the Rose Bengal Test (RBT) and the buffered plate agglutination test (BPAT), as well as the ELISA and the FPA, are sufficient screening tests for control at the national or local level28. If necessary, positive reactions can be retested using an appropriate confirmatory strategy.
Agglutination test
Serological diagnosis of first completed through an agglutination test29. The primary agglutination antibodies IgM and IgG2 detected through these tests similar to serum agglutination test (SAT)30. Due to cross-reaction by IgM antibodies created in the competition of sequences and other closely to Brucella species, therefore, its sensitivity is good, and specificity is low31. This test was rejected for international trading.
Antiglobulin (Coombs) test
The direct Coombs test is also known as the direct antiglobulin test (DAT) was the first time discovered by Coombs, Mourant, and Race in 1945 and is still an essential assay for the diagnosis of autoimmune hemolytic anemia (AIHA. The DAT can identify complement (C3) and RBC-bound IgG that opsonizes RBCs32. The serum agglutination test gives negative or suspected results, so a Coombs test used for confirmation of results. Due to the advantage of this test to detect incomplete antibodies of IgG types that combine with cellular antigens, this test is used for the epidemiological study but does not increase agglutination reaction (
The 2-mercaptoethanol test
The 2-MET are two forms that use either 2-mercaptoethanol33 or dithiothreitol34. Dithiothreitol has recommended, because of the toxicity of 2-mercaptoethanol. The disulfide of IgM is being condensed to the manometric molecule and unable to agglutination essentially calculate IgG unable to agglutinate. However, IgG can also be decreased in the procedure, providing false-negative results (
Buffered plate agglutination test (BPAT)
The BAPT test was developed to detect Brucella spp antibody. BPAT is an easy cheap and uniform agglutination test. It utilized antigen at pH of 3.65, which is prepared from S119.3 whole cells dyed with crystal violet and brilliant green colors. The test is responsible for false-positive results because of the prozoning effect and vaccinal antibodies36. Due to the reduction of non-specific test reactions, this test is very beneficial. It has directed for IgG testing37.
Brucellin allergic skin test (BAST)
The skin test is an allergic test that measures Brucella spp's unique cellular immune response. Brucellin allergic skin test (BAST) based on a delayed-type hypersensitivity reaction with a maximum sensitivity at 72 hours post-inoculation. This delayed type of hypersensitivity reaction is measured at the site of inoculation by the increase in skin thickness. The test is specific to complement serological tests for the diagnosis of bovine , and thus decrease the figure of false-positive reactions significantly by distinguishing from other cross-reacting organisms27. The test is more specific to RBPT and CFT in conditions of its specificity (exceeding 99%). The skin test is highly specific, but its weak sensitivity makes it a good herd test, but not an individual certification test. Thus, it is often suggested for use at the herd level as a positive test in unvaccinated animals38.
Complement fixation tests
The IgM isotypes incompletely damaged during the inactivation process, so the CFT test mostly detects the IgG isotypes antibody. After the IgM type, the antibodies IgG1 types usually appear. The SAT and CFT best performed the control and surveillance of the disease. The test indicates an association with the recovery of Brucella from artificial recovery or naturally infected animals. Although the test is rapid and precise, it does not permit differentiation between antibodies due to infection from vaccinal antibodies39 (
Comparisons of different diagnostic techniques
| Techniques | Advantage | Disadvantage |
|---|---|---|
| Serum agglutination test | Safe, inexpensive, and appropriate for primary screening | Cross-reactivity with other microorganisms,false-negative results in the early stages of infection, andprozone phenomenon |
| ELISA | Highly sensitive and specific, rapid, simple, and capableof distinguishing between acute and chronic stages | Cross-reactivity |
| Conventional culture | Gold standard and specificity | Time consuming, insensitive or low sensitive, and posinga risk for laboratory staff |
| Coombs antiglobulin agglutination test | Sensitive for relapsing and chronic brucellosis | Labor-intensive and time consuming |
| Lateral flow assay | Easy, rapid, sensitive, and specific | Expensive and possibility of cross-reactivity |
| Complement fixation test | Sensitive and specific | |
| 2-Mercaptoethano | A confirmatory test that allows selective quantification ofIgG anti-Brucella | Toxicity of mercaptoethanol, the possibility of IgGdegradation by the 2-ME, which may lead to falsenegative results |
| Fluorescence polarization immunoassay | Highly sensitive and specific, and capable ofdistinguishing between acute and chronic stages | Costly, need of trained laboratory technicians, andexpensive equipment |
| Rose Bengal plate agglutination test | Cross-reactivity with the antibodies of othermicroorganisms, false-negative results in the early stagesof infection, and prozone phenomenon | |
| PCR | Rapid and accurate; can be performed on blood, serum,CSF, and other clinical samples; can yield positive resultsas early as 10 days after inoculation | Expensive equipment, genus specific Brucladder has lowdetection limit, and works only on pure cultures |
| Real-time PCR | Highly sensitive, specific, and rapid; can be performed onblood, serum, CSF and other clinical samples | Expensive equipment |
MILK RING TEST (MRT)
Fleischer developed a milk ring test (MRT) in 193742. Fleischer promoted adoption of the serum agglutinations test to identify the accurateness of antibodies against Brucella species in milk named the MRT. It is suggested as a screening test to check is bulk tank milk43. The Milk ring test (MRT) is mainly an agglutination test done by cream or whole milk. Hematoxylin Brucella stained cells are added to milk and incubated to occur the reaction. Through the Fc portion of a fat molecule, the immunoglobulins present in the milk attached to fat globules44. MRT detects the IgM and IgA immunoglobulins. This test may be useful for an individual animal or to pooled milk samples by using the maximum volume of milk, comparative to the pool size3. In the milk ring test, the abnormal milk caused a false adverse reaction due to mastitis, milk from the late lactation, and due to the presence of colostrum45. Due to the low concentration of lacteal antibodies or lacking fat, clustering, factors in milk may also cause a false-negative result. Despite all these problems, the milk ring test is very successful, it is the method of choice in dairy herds, and it is a low-cost screening test as compared to other46.

Milk ring test result.
Primary Binding Assays
Primary binding tests directly measure the interaction of antibodies and antigens while traditional serological tests, such as acidified agglutination tests or complementary fixation tests (CFTs), measure secondary phenomena such as agglutination or complementary activation.
The first binding assay technique developed due to some limitations in conventional methods of Brucella diagnosis. This test can find the humoral antibodies to Brucella species very rapidly and accurately47. Due to a short time of exposure, the vaccine has low efficiency, so it eliminates very soon by the immune system, but when a natural antigen enters the host has long exposure and has high energy and not removed by the immune system48. Therefore, to defeat this problem, the fluorescent polarization assay (FPA) and a competitive enzyme-linked immunosorbent assay were developed (cELISA). These tests can differentiate vaccinated animals or animals affected by cross-reacting microorganisms like Escherichia coli O: 116 and O: 157, Salmonella Urbana O: 30, and Yersinia enterocolitica serotype nine from naturally- infected animals. Because of these capabilities, it is possible to decrease the amount of false-positive reactions49.
Lateral Flow Assay (LFA)
The simplified ELISA technique known as lateral flow assay (LFA) is used to detect antibodies of a specific antigen in samples of blood, serum, and milk. The method based on the attachment of antibodies specified to immobilized antigen on a strip (cellulose membrane matrix) that is involved in detecting specific IgM and IgG antibodies in all stages of the diseases3. The main advantage of this technique that it does not require any electrical equipment, but the only refrigerator is used to store the test kits, and this technique is limited in the formation of visible bands because of many ingredients in reaction50.
Fluorescence Polarization Assay (FPA)
Fluorescence Polarization Assay (FPA) is a homogeneous immunoassay. Homogenous immunoassays are single-step assays that do not require repeated washing steps to remove unbound reactants as with conventional primary binding assays.This technique works on the principle of excitation of fluorescent molecules using polarized light to emit it, the emission of light in the solution is inversely proportional to the rotation speed of the molecules. This speed is associated with the viscosity of the solution, temperature and gas constant, and molecular volume51. In the serology of , a component of O-polysaccharide (OPS) of smaller molecular weight is labeled with fluorescein isothiocyanate to use as an antigen. In different samples of serum, milk, and blood if antibodies are present, they are rotated at a reduced rate because of presences of antibodies52.
Competitive Immunoassays
This technique applied by using monoclonal antibody having a high affinity to antigen as compared to a cross-reacting antibody. This technique is mainly used because of its high specificity and involved in the detection of antibody isotypes (IgM, IgG1, IgG2, and IgA). The limitation related to this technique is less sensitive than direct immunoassay53.
Rose Bengal Plate Test (RBPT)
This test is mostly used to diagnose in sheep, goats, and buffalo, and it was the first time used by Morgan for -infected animals. It is an internationally recommended test for screening of detection in animals. The result obtained in a short time, but the limitation of this test is the sensitivity and specificity of RBPT antigen because of its cross-reactivity with other bacterial species such as O157, Vibrio cholera, and some Salmonella spp. The RBPT is spot agglutination technique, which we also called card test or buffered brucella antigen test27. In this test suspension of , smooth cells are retained with Rose Bengal dye using a buffer of Ph 3.65. Low Ph is used to increase the sensitivity of test53. The test can also be used to show the presence of IgM, IgG1, and IgG2 antibodies at neutral PH. This test may result in false-negative results, but it also results in false-positive results due to the significant part to reactions with IgM in animals with the previous vaccination. However, this test occurs actively right when the organisms are not vaccinated previously, and the animal exposed to species.
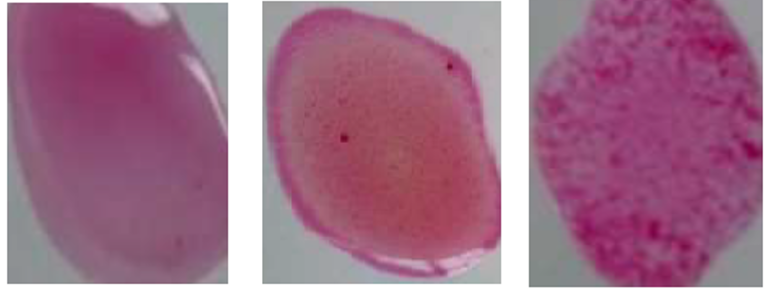
Rose Bengal plate indicating agglutination. Right strong agglutination, moderate, noagglutination.
Conclusion
The diagnosis of in humans and livestock is not an easy task. The “gold standard” of identification is the recovery of the agent from the host, but it is time consuming and laborious method. Which can be done in highly equipped laboratories. For the diagnosis of serological test has been developed more than a century ago, but still, a comprehensive test has not been established. The traditional serological procedure for the diagnostic of is based on the recognition of antibodies, specific to surface LPS. Which is responsible for the low specificity of the test results. An alternative way to solve this problem is the identification of antibodies to specific proteins. It appears that there are no sole immunodominant proteins, but to date, proteomic techniques permit analysis of whole proteome to determine a series of such proteins. The systemic biology methods may not only effectively use in the diagnosis of , but can also develop the understanding of fundamental biological processes in the infected body, including those leading to the large variability in the immune response. The molecular diagnosis method is the most commonly used for the diagnosis of disease. Because it is cost-effective, safe, and rapid as compared to bacteriological tests. PCR-base techniques for the identification of in biological samples are becoming an essential tool for the diagnosis of at biovar and species levels. Although, PCR analysis of the sample should be fully authenticated earlier, the daily use in laboratory testing for . For the detection of DNA, the most promising method is real-time multiplex PCR. Also, the next-generation techniques can be used for organism diagnosis. Still, they are costly but becoming more accessible and popular. Recently, for the recognition and genotyping of the mass spectrometry approach was recommended. This method provides reliable and fast identification of organisms at the species level, but it needed special sophisticated equipment, which is only available in big laboratories. All of the above methods can be very accurate and sensitive, but they can't be utilized in the field condition such as farms, where laboratory testing is available. Meanwhile, these are more suitable for the detection of humans but not in livestock.
Therefore, we believe that the development of a diagnostic test for is associated with an easy-to-use, quick test for initial diagnosis and high sensitivity and specific method for further laboratory testing.
Abbreviations
BBATs: buffered Brucella antigen tests
BPAT: buffered plate agglutination test
FPA: Fluorescence polarization assay
i-ELISA: enzyme-linked immunosorbent assay
ITC: transcribed spacer region
LFA: lateral flow assay
MRT: milk ring test
MTC: Mycobacterium tuberculosis complex
PCR: polymerase chain reaction
qRT: quantitative real-time
RBPT: Rose Bengal Plate Test
RBT: Rose Bengal Test
SAT: serum tube agglutination test
ZN: Ziehl-Neelsen
Conflict of Interest
The author shows no conflict of interest.
Authors' Contributions
All the authors have equally contributed to this work.

