Bio-Electro-Fenton: a novel method for treating leachate in Da Phuoc Landfill, Vietnam
- Faculty of Environment, University of Science, Vietnam National University - Ho Chi Minh City
Abstract
Introduction: Leachate is a noticeable pollution problem because it contains a considerable amount of persistent organic pollutants (POPs). If leachate isn’t treated thoroughly, its leak will negatively affect the environment. Therefore, appropriate treatment technologies are required to remove them. Bio-Electro-Fenton (BEF) is a new method using microorganisms such as electrolytes to convert chemical energy into electricity to help create H2O2 support advanced oxidation process (AOPs). The potentiality of the applicability of BEF technology as a pretreatment step for leachate from Da Phuoc landfill (operation time > 12 years), Ho Chi Minh City. Methods: The BEF pilot scale model (30 x 10 x 10 cm) is divided by a proton exchange membrane (PEM) (Nafion®112) into two chambers (anode and cathode). Cathode chamber used a graphite electrode, the anode chamber used a carbon fabric electrode. The experiments aimed to determine the optimal conditions of parameters affecting the BEF system by determining the efficiency of COD removal and BOD5/COD ratio in leachate. Results: At optimal conditions of the model including pH 3, [Fe2+] = 4g/L, current intensity = 1A, reaction time 60 minutes and airflow = 12 L/min, as a result COD was reduced by 68.2% from 4950 mgO2/L to 1574.1 mgO2/L, the ratio of BOD5/COD = 0.1. Conclusion: The study result showed that Bio-electro-Fenton process is effective for wastewater with high concentrations of pollutant and difficult to treat as leachate, suggesting that the appropriate method for pre-treatment processes support the thorough elimination of pollutants.
Introduce
The population explosion and industrialization in recent years have increased the demand for consumption of goods, materials, and energy which leads to a rapidly increasing amount of domestic waste generated. According to the estimation of of The Ministry of Natural Resources and Environment, the amount of domestic solid waste in urban areas increases by an average of 10 - 16% per year. The majority of domestic waste in Vietnam has been treated by the landfill method. When using this method, a considerable amount of leachate will be generated, which does harm to the environment because leachate contains heavy metals, ammonium, and POPs. The composition and characteristics of leachate are complicated by seasonal changes and burial time hence leachate treatment is extremely sophisticated.
There are many different methods for treating leachate such as flocculation, adsorption, oxidation, etc. AOPs are the most outstanding treatment method which form hydroxyl (OH) free radicals to decompose organic pollutants base on characteristics such as non-selective pollutants oxidation, easily react at room temperature. Typical AOPs such as tradition Fenton and Electro-Fenton have been proven to efficiently treat leachate. However, the traditional Fenton process requires the supply of a large amount of HO and Fe. Besides the amount of reagent added is unstable and requires partial treatment of the chemicals remain after the reaction. For Electro-Fenton, the process requires large energy for the generation of HO . To deal with these disadvantages, it is necessary to progress a low-cost Fenton process which ensures treatment efficiency, therefore BEF method was invented. The BEF method uses microorganisms to decompose organic matter creating energy to form HO for Fenton processes. As a result of the BEF method is a multipurpose method which save costs by reducing electrical energy consumption and using chemicals.
The BEF is a completely new technology. In Vietnam, there has not been any previous article about BEF application in wastewater treatment. Therefore, in this study, we optimize the parameters affecting the BEF method in a complex matrix, with the purpose of evaluating the applicability of the method in leachate treatment. The experiments had been conducted to find optimal conditions through COD treatment efficiency and BOD/COD ratio of leachate.
Materials and methods
Sampling
Samples had collected at the Da Phuoc Solid Waste Treatment Complex in Ho Chi Minh City in March 2019. Sampling, transportation, and preservation techniques complied with TCVN 5999: 1995. Samples had precipitated and stored in 2 plastic containers 30L.
Composition, characteristics of Da Phuoc leachate
| Parameter | Unit | Result | Viet Nam National Technical Regulation – 25:2009/MONRE Column B1 |
| pH | - | 7.8 | - |
| BOD5 | mgO2/L | 1500 ± 59.7 | 100 |
| COD | mgO2/L | 4950 ± 14 | 400 |
| BOD5/COD | - | 0.3 | - |
| Total Phosphor | mg/L | 12.4 ± 0.43 | - |
| [Fe] total | mg/L | 44.1 ± 2.24 | - |
| Total Kjeldahl Nitrogen | mg/L | 1477 ± 62.6 | 60 |
| Ammonium | mg/L | 589.67 ± 22.11 | 25 |
| Total Suspended Solid | mg/L | 15.3 | - |
According to
BEF pilot system and operation
BEF pilot scale model (30 x 10 x 10 cm) divided by a PEM (Nafion 112) into two chambers. The volume of each chamber (anode and cathode chambers) was 1.5 L with a working volume of 1.134 L. Cathode chamber used a graphite electrode, the anode chamber used a carbon fabric electrode with size (7.5 x 5 x 0.4 cm). The electrodes connected with copper wire of 2 mm diameter and 40 cm length through an external transistor of 100 Ω and DC supply with voltage 0 - 30V, current 0 - 5A to adjust to each test requirement (Figure 1a, c).

(a) Photograph (b) Electro transfer mechanism in the BEF system (c)Schematic drawing of the experimental setup of the BEF system
At the first stage of the survey, anode chamber was loaded with anaerobic sludge and 500 mL of artificial wastewater (glucose 30 g/L, KH PO 4.33 g/L, NaHPO 2 g/L, NHCl 0.2 g/L, KCl 0.13 g/L)1 to help anaerobic microorganisms grow and develop stably. The microorganisms decompose glucose to H and produce electrons (
Then ion H passed through the PEM to the cathode chamber. Due to the potential difference in voltage, electrons formed at the anode chamber will transfer the external resistor and to the cathode electrode. In the cathode chamber, the pump supplies oxygen for the reaction (
All chemicals and reagents used for the experiments were of analytical grade and supplied by Merck (Germany). In each experiment, 500 mL of leachate was added at the cathode chamber, pH was adjusted using NaOH 1N, HSO 1N. The agents used in the Fenton process includes FeSO.7HO 5%. All experiments were performed at 30C temperature and atmospheric pressure in a batch mode manner. Data are representative with three replicates and their average are reported.
Chemical analysis
pH value of wastewater was measured by Schott - LAB 850 - Germany. The parameters COD, BOD, Total Phosphor, Total Nitrogen, and TSS were determined according to the Standard Methods for the examination of water and wastewater2.
COD, BOD were observed throughout the experiment. For the Bicromate method, the amount of residual HO and Fe in sample after the reaction affect the results of COD determination according to the reaction (
In this study, reaction in sample after treatment will be stopped immediately by adding NaOH 2.5 N to pH 10 - 11 to precipitate iron, then heat at 70-80C within 30 minutes to completely remove residual HO before conducting COD analysis4.
Data processing
- Processing efficiency (H%) is calculated according to the formula:
Where: C is the initial concentration (mg/L)
C is the final concentration (mg/L)
Results
To determine the maximum treatment efficiency of the method for leachate as well as evaluate the influence of important parameters in the BEF model, experiments are performed to optimize each parameter in a complex matrix of effects. The result show similarity to other Fenton processes, the optimal pH of BEF is at 3 (Figure 2).

COD removal efficiency (%) (
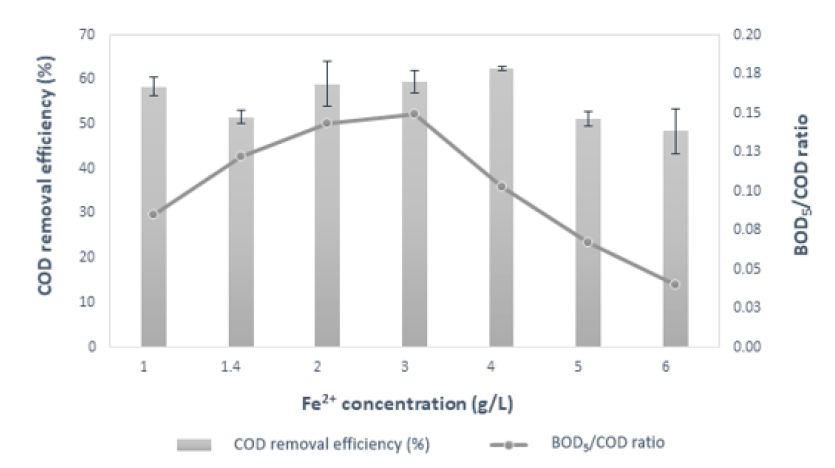
A larger concentration of Fe catalyst will increase COD treatment efficiency significantly, up to 4 g/L. However, when concentration is too high, it will reduce processing efficiency. Treatment efficiency tends to decrease at increasing iron concentration (Figure 3). The COD removal efficiency decreased from 54.82 2.04 % to 51.22 1.53 %, while the BOD/COD ratio increased when concentration [Fe] increased from 1 g/L to 1.4 g/L, respectively. When the concentration [Fe] is from 1.4 g/L to 4 g/L, the treatment efficiency increases linearly with [Fe]. The BOD/COD ratio reached the highest values of 0.15 at 3 g/L and the lowest value of 0.04 at 6 g/L.

COD removal efficiency (%) (
For current intensity, the addition of an external current to the system helps to accelerate the Fenton process due to electrons are increased with electrons made from anaerobic organisms decompose glucose in the anode chamber. The optimum current for the system is recorded at 1 A (Figure 4).

COD removal efficiency (%) (
The reaction time and airflow rate provided to the system were also surveyed. (Figure 6, Figure 5) The reaction time of 60 minutes, the airflow rate of 12 L/min yiel ded the highest COD removal efficiency, reaching 62.42 0.99 % and 68.20 1.04 %, respectively.

COD removal efficiency (%) (

COD removal efficiency (%) (
Discussion
Effect of pH on the Bio-Electro-Fenton process
Both of case pH is too low and too high are effect to the efficiency of the Fenton process4. The Fenton process can be inhibited because of very low pH values (pH < 3). Fe , which exists as Fe[HO] , has a slower reaction rate with HO than Fe[HO] at pH = 3 which leads to less hydroxyl (OH) generation5. Besides, when the pH is too low H will react with HO to form peroxone (H O) according to the reaction HO + H → HO. The peroxone do not react with Fe therefore it will decrease OH formation efficiency which leads to reduces the processing efficiency of the Fenton process.
At high pH (pH ≥ 4), the formation of ferrous/ferric hydroxide complexes leads to the catalyst deactivation, which decreases the quantity of OH6. In addition, the decomposition of HO into HO and O also increases with increasing pH8, 7. The accumulation of protons due to the slow and insufficient protons diffusion through membrane would cause a decrease of pH in anode chamber6. Therefore, the cathode electrode material as a source to self-regulate the supply of Fe under neutral conditions is necessary to reduce the cost of pH adjusting chemicals.
Effect of [Fe] on the Bio-Electro-Fenton process
Fe is an extremely important factor that directly affects the Fenton process. Considering that the HO production rate and yield could be constant at defined conditions in bio-electro-chemical system, Fe as catalyst could be a key factor for the final treatment performance9. As a result, a certain amount of Fe saves chemicals and makes the process more efficient.
At low [Fe ] concentration, hydroxyl (OH) produced just enough to oxidize the biodegradable organic compounds. When the dose of Fe increased, amount of hydroxyl is more produced. The oxidation of organics withOH occurs through well-known pathways, principally H atom abstraction (mainly from aliphatics) and addition to C = C bonds (mainly with aromatics leading to the formation of hydroxylated aromatic derivatives)10. The persistent organics changes into a biodegradable form, increasing the BOD value, increasing the BOD/COD ratio and leading to a reduction in processing efficiency.
At higher concentration [Fe], from 4 g/L to 6 g/L, treatment efficiency decreases due to reduction of radicalsOH according to the reaction (
In addition, the Fe ions formed can react with HO to reduce the mineralization of organic substances (
Excess iron salts increase the amount of dissolved salt (TDS) and conductivity. Besides, after stopping the reaction, treated wastewater must be adjusted to neutral pH. pH raising create a large amount of iron deposits in the sludge12.
Effect of current intensity on the Bio-Electro-Fenton process
The current intensity produced by the microorganism system in the cathode chamber to create HO, which is an extremely important catalyst in the BEF. Higher current will increase the amount of HO , thus increasing the number of (OH) hydroxyl radicals in the electrolyte environment. However, in the experiments, the efficiency of the current generation by the microorganism system was quite low, therefore to increase the processing efficiency, the experiment used an external power (DC) to connect the BEF system to supplement the process. The technology converts from microbial electrolysis cells (MEC) to the microbial fuel cell (MFC). Besides, increasing the current too high will affect processing efficiency while wasting a significant amount of energy. In this case, optimized current intensity is usually chosen to attain the maximum HO production rate and yield, and its value quite depends on the cathode material used6.
The current increased (0,5 to 1A) with the increasing rate of pollutant degradation (49.60 2.17 % to 62.42 1.81 %) since more H O are formed at a given time. However, the COD concentration decreased as the current increased above 1A, the efficiency decreased from 62.42 1.81 % to 50.75 1.72 % at 2.5A. The current cannot be increased indefinitely since the cathode potential would be changed by the applied voltage, resulting in the side reactions, and thereby decreasing current efficiency and pollutants removal efficiency. The side reactions can involve: (i) high current density by adding high external voltage would enhance the HO electrochemical reduction through (

Samples of current intensity after processing.
Effect of reaction time on the Bio-Electro-Fenton process
The time needed to complete a Fenton reaction will depend on the many variables such as catalyst dose and wastewater strength. For more complex or more concentrated wastes as leachate, the reaction in various studies fluctuated between 30 minutes and 3 hours13.
COD removal efficiency increased gradually and reached the highest of 62.42 0.99 % at 60 minutes, BOD in leachate also increased from 50 mgO/L to 191 mgO/L. The complex organic is decomposed into simpler organic substances, thus reduced COD concentration, increased BOD concentration in wastewater, contributed to an increase BOD/COD ratio. Increasing the reaction time will create more OH radicals to form HO, while also fostering the Fenton reactions occur more to thoroughly oxidize the pollution.
After the equilibrium time, COD concentration decreased, the BOD/COD ratio did not change significantly. The BOD concentration raised, this can be explained by the persistent organic pollutant degradation which still continues to occur but tends to slow down. In addition, intermediates created are more difficult to oxidize, which inhibits the Fenton reaction. The amount of Fe when being regenerated will be oxidized to FeO, thus reducing treatment efficiency4.
Effect of airflow on the Bio-Electro-Fenton process
Oxy is one of the key parameters in the BEF system, which is the electron acceptor in the cathode chamber to produce HO. High airflow rate could enhance the dissolved oxygen in solution and promote the oxygen mass transfer rate, and thus, is beneficial for HO production and accumulation in bioelectrochemical systems6. It is important to set the optimal airflow rate because if the speed is too low, it will not maintain enough dissolved oxygen and if the speed is too high, it will cost a lot of operating energy for the system.
COD treatment efficiency increased with increasing airflow rate and reached 68.20 1.04 % (Figure 6) at the maximum airflow rate of 12 L air/minute. The reason is that the excessive high airflow rate could also disturb the mass transfer between catholyte and electrode and lead to a low catalytic efficiency for pollutants degradation by the Fenton process6. The ratio of BOD/COD also increased significantly due to (OH) hydroxyl free radicals non-selectively reacts with POPs compounds to create more easily biodegradable compounds.
However, when continuing to increase the airflow rate, from 12 to 16 L air/minute, COD treatment efficiency decreased from 68.20 1.04 % to 62.22 1.12 %, respectively. This is explained by the extremely high airflow rate which leads to a chemical imbalance of reaction (2) O + 2H + 2e → HO and reduces the accumulation of HO.
Conclusions
The results obtained in the study show that the use of the Bio-electro-Fenton process is effective for wastewater with large concentrations and difficult to treat as leachate. At optimal conditions of the model including pH 3, [Fe] = 4g/L, current intensity = 1A, reaction time 60 minutes and airflow = 12 LO/min, as a result COD from 4950 14 mgO/L to 1574.1 51.4 mgO/L (decreased 68.2 1.04 %). The ratio of BOD/COD decreased from 0.3 to 0.1 due to Fenton reactions which reduced a large amount of easily biodegradable organic matter, suggesting that the appropriate method for pre-treatment processes support to thoroughly eliminate pollutants.
List of abbreviations
AOPs: Advanced oxidation process
BEF: Bio-electro-Fenton
PEM: Proton exchange membrane
POPs: Persistent organic pollutants
Authors’ contributions
The author Ho Nhut Linh did the experiment. The author Ho Truong Nam Hai discussed the results and wrote the final manuscript. All authors approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.

