Synthesis and evaluation of α-glucosidase and tyrosinase inhibitory activities of ester derivatives of usnic acid
- Department of Chemistry, Ho Chi Minh City University of Education, District 5, Ho Chi Minh City, Viet Nam
- Institute of Fundamental and Applied Sciences, Duy Tan University, District 1, Ho Chi Minh City, Viet Nam
Abstract
Introduction: Usnic acid isolated from lichen was a potential bioactivity compound. It has a broad spectrum bioactivity, including antiviral, anti-inflammatory, anticancer… However, low solubility in water limited its application. Many researchs have done to overcome the restriction. Recent results showed that usnic acid derivatives bearing triazole, enamine, pyrazole and benzylidene groups had strong antiviral and anticancer activities. Thus, investigation of usnic acid derivatives synthesis was an attractive aspect due to the diversity of bioactivities of usnic acid derivatives.
Methods: Usnic acid was isolated from lichen, six ester derivatives of usnic acid were synthesized from usnic acid with acetyl chloride and benzoyl chloride under stirring at room temperature. The products were evaluated α-glucosidase and tyrosinase inhibitory activities.
Results: All the ester derivatives were created with good yields. All derivatives exhibited the same or higher activity comparing with usnic acid. Ester of usnic acid bearing benzoyl group showed excellent α-glucosidase activity with IC50 26.7±0.57 and 68.8±0.15 µM.
Conclusion: Among the ester derivatives, UE1 and UE6 were reported as as new compounds. Interestingly, all products displayed the same or higher biological activity than the starting material, usnic acid when evaluated against α-glucosidase and tyrosinase.
INTRODUCTION
Isolated compounds from lichens exhibited a wide range of biological properties, such as antimicrobial, antiviral, anti-inflammatory, anticaner…1. Usnic acid, a dibenzofuran derivative found only in lichens was a remarkable substance. Usnic acid has a broad spectrum of bioactivity, especially against gram-positive bacteria such as and antifungal 2. Futhermore, it also has antiviral, anti-inflammatory, antipyretic… activities 2. experiments showed that usnic acid could inhibit many human cancer cell lines growth 3. However, toxicity with liver and low solubility in water of usnic acid has limited application of it in cancer treatment. This attracts interests of many researchers to overcome the limit.
The first research of usnic acid derivatives synthesis was carried out by Takai in 1979, the solubility of products were improved by preparing glycoside and imine derivatives of usnic acid 4. Recently, many researchs showed that usnic acid bearing triazole, enamine, pyrazole and benzylidene groups had strong antiviral and anticancer activities 5, 6, 7, 8. The diversity of bioactivities of usnic acid derivatives showed that they could be a potential drugs in medicinal treatments. Herein, we described a procedure of ester derivatives synthesis from usnic acid, these compounds were evaluated of and inhibitory activities.
MATERIALS AND METHODS
Materials
(+)-Usnic acid isolated from lichen.
Acetyl chloride, benzoyl chloride (Sigma-Aldrich).
Silica gel 60 (HiMedia, India).
Bruker Advance III (400 MHz for H NMR and 100 MHz for C NMR) spectrometer with TMS as internal standard recorded NMR spectra.
The HR–ESI–MS were recorded on a HR–ESI–MS Bruker microTOF Q-II.
Column chromatography was performed with silica gel 60.
General experimental procedure
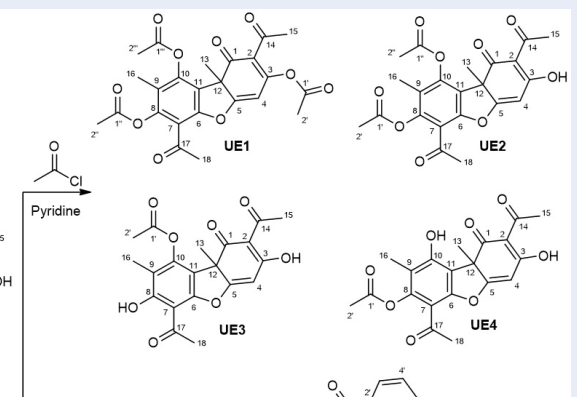
A mixture of (+)-usnic acid (0.250 g, 0.727 mmol) in CHCl (5.0 mL) was stirred at room temperature for 5 minutes. Acetyl chloride (0.341 g, 4.350 mmol) was added, followed by pyridine (3.5 mL, 43.502 mmol) and stirred at room temperature for 6 h. Then, the organic layer was extracted with water and saturated with aqueous NaHCO, respectively, and dried over anhydrous NaSO. The mixture was filtered and evaporated using rotatory vacuum evaporator. The products, UE1-4 were purified by subjecting to silica gel column.
A mixture of (+)-usnic acid (0.250 g, 0.727 mmol) in CHCl (5.0 mL) was stirred at room temperature for 5 minutes. enzoyl chloride (0.611 g, 4.350 mmol) was added, followed by pyridine (3.5 mL, 43.502 mmol) and stirred at room temperature for 6 h. The products, UE5 were purified by subjecting to silica gel column.
A mixture of UE3 (0.280 g, 0.727 mmol) in CHCl (5.0 mL) was stirred at room temperature for 5 minutes. enzoyl chloride (0.611 g, 4.350 mmol) was added, followed by pyridine (3.5 mL, 43.502 mmol) and stirred at room temperature for 6 h. The products, UE6 were purified by subjecting to silica gel column.
Biological activities investigation
These inhibitory activities were evaluated according to 9. Enzymatic activity was calculated by measuring absorbance at 405 nm (ALLSHENG micro plate reader AMR-100). All samples were analyzed in triplicate at various concentrations to obtain the IC value of each compound. The mean values and standard deviation were also identified.
Structure determination of products
The products were verified structures by H and C NMR method using CDCl as solvent and HR-ESI-MS method.
UE1: Light yellow powder, m = 0.0342 g, yield: 10 %; H NMR (CDCl, 400 MHz) δ 6.38 (1H, s), 2.65 (3H, s), 2.40 (3H, s), 2.35 (3H, s), 2.23 (3H, s), 2.22 (3H, s), 2.19 (3H, s), 2.02 (3H, s). C NMR (CDCl, 100 MHz) δ 203.0, 202.9, 195.0, 169.1x2, 168.5, 151.2, 147.8, 145.7, 145.5, 144.5, 121.5, 120.3, 115.5, 113.7, 108.5, 47.0, 31.8, 29.5, 21.1, 20.7, 20.5, 9.7, 9.2. HR-ESI-MS m/z [M+H] calcd. for CHO : 471.1291; found: 471.1297.
UE2: Light yellow powder, m = 0.1055 g, yield: 34 %; H NMR (CDCl, 400 MHz) δ 5.90 (1H, s), 2.60 (3H, s), 2.54 (3H, s), 2.46 (3H, s), 2.33 (3H, s), 1.98 (3H, s), 1.81 (3H, s). C NMR (CDCl, 100 MHz) δ 198.6, 195.0, 192.8, 190.9, 177.8, 168.9, 168.8, 153.7, 149.0, 148.5, 123.6, 118.9, 116.1, 106.2, 98.8, 59.5, 32.1, 31.1, 26.2, 21.4, 20.8, 10.4.
UE3: Light yellow powder, m = 0.0420 g, yield: 15 %; H NMR (CDCl, 400 MHz) δ 13.22 (1H, s), 5.91 (1H, s), 2.74 (3H, s), 2.54 (3H, s), 2.45 (3H, s), 2.03 (3H, s), 1.78 (3H, s). C NMR (CDCl, 100 MHz) δ 201.9, 198.4, 193.3, 190.9, 178.1, 168.6, 163.3, 155.7, 151.5, 117.7, 111.1, 106.3, 105.4, 98.8, 59.4, 32.0, 31.2, 26.0, 21.4, 9.3.
UE4: Light yellow powder, m = 0.0505 g, yield: 18 %; H NMR (CDCl, 400 MHz) δ 11.07 (1H, s), 5.97 (1H, s), 2.66 (3H, s), 2.57 (3H, s), 2.35 (3H, s), 2.06 (3H, s), 1.80 (3H, s). C NMR (CDCl, 100 MHz) δ 201.9, 197.8, 194.0, 191.8, 179.3, 169.2, 155.5, 154.2, 149.7, 117.4, 110.0, 109.9, 105.4, 98.5, 59.1, 32.4, 32.0, 28.0, 20.9, 8.9.
UE5: Light yellow powder, m = 0.3250 g, yield: 81 %; H NMR (CDCl, 400 MHz) δ 13.32 (1H, s), 10.52 (1H, s), 8.01 (2H, d, = 8.0 Hz), 7.88 (2H, d, = 8.0 Hz), 7.66 (2H, t, = 8.0 Hz), 7.53 (2H, t, = 8.0 Hz), 7.46 (1H, t, = 8.0 Hz), 7.32 (1H, t, = 8.0 Hz), 6.03 (1H, s), 5.43 (1H, d, 1.2), 5.24 (1H, d, 1.2), 2.65 (3H, s), 2.12 (3H, s), 1.88 (3H, s). C NMR (CDCl, 100 MHz) δ 200.9, 200.5, 174.0, 165.1, 164.5, 164.0, 163.0, 157.5, 156.4, 143.5, 134.6, 133.6, 130.7, 130.1, 128.9, 128.5, 128.4, 127.9, 114.6, 109.8, 109.2, 104.0, 101.9, 96.6, 60.7, 31.3, 31.1, 7.7.
UE6: Light yellow powder, m = 0.2529 g, yield: 71 %; H NMR (CDCl, 600 MHz) δ 8.18 (2H, d, 8.0), 7.66 (1H, t, 8.0), 7.53 (2H, t, 8.0), 5.92 (1H, s), 2.60 (3H, s), 2.56 (3H, s), 2.48 (3H, s) 2.04 (3H, s), 1.85 (3H, s). C NMR (CDCl, 150 MHz) δ 202.5, 198.7, 195.0, 190.9, 177.9, 168.9, 164.6, 153.5, 148.9, 148.5, 134.2, 130.6, 128.9, 128.7, 119.1, 116.7, 114.5, 114.0, 98.9, 59.6, 32.0, 29.8, 26.2, 21.5, 10.6. HR-ESI-MS m/z [M+Na] calcd. for CHONa: 513.1162; found 513.1122.
RESULTS
Figure 1 showed esterification of usnic acid with acetyl chloride and benzoyl chloride. Six ester derivatives (UE1-6) were synthesized from usnic acid.

Esterification of usnic acid
Ester derivatives synthesis of usnic acid
| Entry | Ester compound | Yield (%)a |
| 1 | UE1 | 10 |
| 2 | UE2 | 34 |
| 3 | UE3 | 15 |
| 4 | UE4 | 18 |
| 5 | UE5 | 81 |
| 6 | UE6 | 71 |
1H NMR data of ester derivatives
| Position | Usnic acid(δH J, Hz) | UE1(δH J, Hz) | UE2(δH J, Hz) | UE3(δH J, Hz) | UE4(δH J, Hz) | UE5(δH J, Hz) | UE6(δH J, Hz) |
| 1 | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - | - |
| 3 | - | - | - | - | - | - | - |
| 4 | 5.97 s | 6.38 s | 5.90 s | 5.91 s | 5.97 s | 6.03 s | 5.92 s |
| 5 | - | - | - | - | - | - | - |
| 6 | - | - | - | - | - | - | - |
| 7 | - | - | - | - | - | - | - |
| 8 | - | - | - | - | - | - | - |
| 9 | - | - | - | - | - | - | - |
| 10 | - | - | - | - | - | - | - |
| 11 | - | - | - | - | - | - | - |
| 12 | - | - | - | - | - | - | - |
| 13 | 1.76 s | 2.02 s | 1.81 s | 1.78 s | 1.80 s | 1.88 s | 1.85 s |
| 14 | - | - | - | - | - | - | - |
| 15 | 2.66 s | 2.40 s | 2.54 s | 2.54 s | 2.57 s | 5.43 d (1.2)5.24 d (1.2) | 2.56 s |
| 16 | 2.11 s | 2.35 s | 2.46 s | 2.45 s | 2.35 s | 2.12 s | 2.48 s |
| 17 | - | ||||||
| 18 | 2.68 s | 2.65 s | 2.60 s | 2.74 s | 2.66 s | 2.65 s | 2.60 s |
| 3-OH | - | - | - | - | - | - | - |
| 8-OH | 13.29 s | - | - | 13.22 s | - | 13.32 s | - |
| 10-OH | 11.01 s | - | - | - | 11.07 s | 10.52 s | - |
| 2’ | 2.23 s | 2.33 s | 2.03 s | 2.06 s | - | - | |
| 2” | 2.22 s | 1.98 s | - | - | 2.04 s | ||
| 2”’ | 2.19 s | - | - | - | - | - | |
| 3’,7’ | 8.01 d (8.0) | 8.18 d (8.0) | |||||
| 3”,7” | 7.88 d (8.0) | - | |||||
| 4’-6’ | 7.66 t (8.0) | 7.66 t (8.0) | |||||
| 4”-6” | 7.53 t (8.0) | - | |||||
| 5’ | 7.46 t (8.0) | 7.53 t (8.0) | |||||
| 5” | 7.32 t (8.0) | - |
13C NMR data of ester derivatives
| Position | Usnic acid (δC) 9 | UE1 (δC) | UE2 (δC) | UE3 (δC) | UE4 (δC) | UE5 (δC) | UE6 (δC) |
| 1 | 198.1 | 195.0 | 192.8 | 193.3 | 194.0 | 200.5 | 195.0 |
| 2 | 105.3 | 120.3 | 118.9 | 111.1 | 110.0 | 109.2 | 116.7 |
| 3 | 191.7 | 151.2 | 190.9 | 190.9 | 191.8 | 165.1 | 190.9 |
| 4 | 98.3 | 108.5 | 98.8 | 98.8 | 98.5 | 96.6 | 98.9 |
| 5 | 179.4 | 147.8 | 177.8 | 178.1 | 179.3 | 174.0 | 177.9 |
| 6 | 155.2 | 145.5 | 149.0 | 155.7 | 154.2 | 156.4 | 148.5 |
| 7 | 101.6 | 113.7 | 106.2 | 105.4 | 105.4 | 101.9 | 114.0 |
| 8 | 163.9 | 145.7 | 153.7 | 163.3 | 155.5 | 157.5 | 153.5 |
| 9 | 109.4 | 121.5 | 123.6 | 117.7 | 117.4 | 114.6 | 119.1 |
| 10 | 157.5 | 144.5 | 148.5 | 151.5 | 149.7 | 143.5 | 148.9 |
| 11 | 103.9 | 115.5 | 116.1 | 106.3 | 109.9 | 104.0 | 114.5 |
| 12 | 59.1 | 47.0 | 59.5 | 59.4 | 59.1 | 60.7 | 59.6 |
| 13 | 7.5 | 9.2 | 10.4 | 9.3 | 8.9 | 7.7 | 10.6 |
| 14 | 200.3 | 203.0 | 198.6 | 201.9 | 201.9 | 163.0 | 202.5 |
| 15 | 27.8 | 29.5 | 31.1 | 31.2 | 32.0 | 109.8 | 29.8 |
| 16 | 32.2 | 9.7 | 26.2 | 21.4 | 28.0 | 31.1 | 26.2 |
| 17 | 201.7 | 202.9 | 195.0 | 198.4 | 197.8 | 200.9 | 198.7 |
| 18 | 31.2 | 31.8 | 32.1 | 32.0 | 32.4 | 31.3 | 32.0 |
| 1’ | 169.1 | 168.9 | 168.6 | 169.2 | 164.5 | 168.9 | |
| 1” | 169.1 | 168.8 | 164.0 | 164.6 | |||
| 1’” | 168.5 | - | - | - | |||
| 2’ | 21.1 | 21.4 | 26.0 | 20.9 | 127.9 | 128.7 | |
| 2” | 20.7 | 20.8 | 128.4 | 21.5 | |||
| 2’” | 20.5 | - | - | - | |||
| 3’ | 130.7 | 130.6 | |||||
| 3” | 130.1 | - | |||||
| 4’ | 128.9 | 128.9 | |||||
| 4” | 128.5 | - | |||||
| 5’ | 134.6 | 134.2 | |||||
| 5” | 133.6 | - | |||||
| 6’ | 128.9 | 128.9 | |||||
| 6” | 128.5 | - | |||||
| 7’ | 130.7 | 130.6 | |||||
| 7” | 130.1 | - |
and inhibitory activities of UE1-6 were listed in

Proposed mechanism of UE3 synthesis from usnic acid
| Entry | Compound | α-Glucosidase IC50 (µM) | Tyrosinase IC50 (µM) |
| 1 | UE1 | >200 | NA |
| 2 | UE2 | >200 | >200 |
| 3 | UE3 | >200 | NA |
| 4 | UE4 | >200 | >200 |
| 5 | UE5 | 26.7 ± 0.57 | >200 |
| 6 | UE6 | 68.8 ± 0.15 | NA |
| 7 | Usnic acid | >200 | NA |
| 8 | Acarbose | 93.6±0.49 | |
| 9 | Kojic acid | 36.1 ± 1.07 |
DISCUSSION
Ester derivatives synthesis from usnic acid
There are three hydroxy groups in usnic acid structure at C-3, C-8 and C-10 could be esterified. In the reaction, we use large amounts of acetyl chloride in order to react at three hydroxy groups completely. However, the reaction produced four ester derivatives (UE1-4) depending on the number and position of hydroxy groups that participated in the reaction when acetyl chloride was used as a reactant. Besides, only one product (UE5) was created when benzoyl chloride was used. Moreover, the ester product (UE6) was also generated when UE3 product reacted with benzoyl chloride in the same conditions (Figure 1). The synthesis results were listed in
The H NMR spectrum of UE1 showed an olefin proton at δ 6.38, and seven methyl groups at δ 2.65, 2.40, 2.35, 2.23, 2.22, 2.19 and 2.02. The C NMR spectrum of UE1 displayed twenty-three carbon signals, including three ketone carbons at δ 203.0, 202.9 and 195.0, three carboxyl carbons at δ 169.1x2 and 168.5, ten olefin carbons in the range of δ 155.0-100.0, one tertiary carbon at δ 47.0 and seven methyl carbons at δ 31.8, 29.5, 21.1, 20.7, 20.5, 9.7 and 9.2. The lack of 8- and 10-OH signal in usnic acid along with the appearance of seven methyl groups (usnic acid has only four methyl groups 10) indicated the esterification reaction occurred on 3-, 8-, and 10-OH of usnic acid. Thus, UE1 is established as 3,8,10-triacetoxyusnic acid.
The H NMR spectrum of UE2 showed an olefin proton at δ 5.90, and six methyl groups at δ 2.60, 2.54, 2.46, 2.33, 1.98 and 1.81. The lack of both of 10-OH and 8-OH in usnic acid along with the appearance of only two acetoxycarbonyl groups (δ 2.33 and 1.98; δ 168.9 and 168.8) indicated the esterification reaction occurred on both of 10-OH and 8-OH of usnic acid. Thus, the structure of UE2, 8,10--diacetylusnic acid 10, is elucidated as shown in Figure 1.
The H NMR spectrum of UE3 showed a singlet of hydroxy chelated signal at δ 13.22, an olefin proton at δ 5.91, and five methyl groups at δ 2.74, 2.54, 2.45, 2.03, and 1.78. Similar to UE2, the lack of 10-OH in usnic acid 10 along with the appearance of only one acetoxycarbonyl group (δ 2.03, δ 168.6 and 26.0) indicated the esterification reaction occurred on 10-OH of usnic acid. Thus, the structure of UE3, 10--acetylusnic acid 11, is elucidated as shown in Figure 1.
The examination of the H and C NMR spectra of UE4 revealed the similar spectra to those of UE3, excepted for the lack of 8-OH and the occurrence of 10-OH that indicated the reaction occurred at 8-OH. Thus, UE4, 8--acetylusnic acid 11, is established as shown in Figure 1.
The H NMR of UE5 displayed the presence of two chelated hydroxyl groups at δ 13.32 and 10.52, ten aromatic protons at δ 7.00-8.50, three olefin protons at δ 6.03, 5.43, and 5.24, and three methyl groups at δ 2.65, 2.12 and 1.88. Comparison with those of usnic acid indicated the hydroxyl groups at δ 13.32 and 10.52 belonging to 8-OH and 10-OH, respectively. Moreover, the appearance of ten aromatic protons at δ 7.00-8.50 ppm along with a couple gem olefin proton at δ 5.43 (1H, d, = 1.2 Hz) and 5.24 (1H, d, = 1.2 Hz) implied the disubstitution on C-14 and C-3. Finally, UE5 is established as benzoic acid 1-(6-acetyl-3-benzoyloxy-7,9-dihydroxy-8,9b-dimethyl-1-oxo-1,9b-dihydro-dibenzofuran-2-yl)-6inyl ester as shown in Figure 111.
The H NMR spectrum of UE6 showed five aromatic protons at δ 8.5-7.5, that implied monobenzoyl chloride reacted with UE3. A singlet signal at δ 5.86 (1H, s), belonging to H-4 in starting material, and five methyl groups at δ 2.60, 2.56, 2.48, 2.04 and 1.85. The examination of the C NMR spectrum revealed some important structural differences from UE3 including the occurrence of five aromatic carbons at δ 134.2, 130.6 x2 and 128.9x2 confirmed the addition of monobenzoyl chloride. Moreover, the lack of chelated hydroxyl proton 8-OH at δ 13.22 (UE3) identificated that the reaction occurred at 8-OH. Finally, the structure of UE6 was established as shown in Figure 1.
Biological activities of usnic acid derivatives
Six usnic acid derivatives including via esterification (UE1-6) were further tested with and inhibitory activities. From the results, all derivatives exhibited the same or higher activity comparing with starting material (usnic acid: >200 µM and no activity (NA) for and , respectively). Especially, UE5 and UE6 showed excellent activity with IC 26.7±0.57, and 68.8±0.15 µM, respectively. These compounds not only displayed higher activity than that of usnic acid, but also with that of a positive control, acarbose (IC: 93.6±0.49 µM) as shown in
CONCLUSION
From usnic acid, six derivatives were synthesized via esterification reactions (UE1-6). Their chemical structures were elucidated by NMR and HRESIMS as well as comparison with those from literature. Among them, UE1 and UE6 were reported as as new compounds. Interestingly, all products displayed the same or higher biological activity than the starting material, usnic acid when evaluated against and . In the assay, UE5 and UE6 showed excellent activity (IC 26.7±0.57, and 68.8±0.15 µM, respectively). On the other hand, all tested compounds revealed weak or no inhibitory activity in the assay.
ABBREVIATIONS
H NMR: Proton nuclear magnetic resonance;
C NMR: Carbon-13 nuclear magnetic resonance;
s: singlet;
d: doublet;
t: triplet.
CONFLICTS OF INTEREST
The authors declare that they have no competing financial interest.
AUTHOR CONTRIBUTION
All authors contributed in conducting experiments, acquisition of data, interpretation of data, searching the bibliography and gave final approval of the manuscript to be submitted.
ACKNOWLEDGEMENT
The authors are indebted to Dr. Warinthorn Chavasiri and Mrs. Asshaima Paramita Devi (Center of Excellence in Natural Products Chemistry, Department of Chemistry, Faculty of Science, Chulalongkorn University, Thailand) for performing the enzyme inhibitory against and .

