Xao tam phan (Paramignya trimera) methanol extract induced apoptosis in hepatocellular carcinoma HepG2 cell line in vitro
- Stem Cell Institute, University of Science Ho Chi Minh City, Viet Nam
- Medical Genetic Institute, Ho Chi Minh City, Viet Nam
- Vietnam National University Ho Chi Minh City, Viet Nam
Abstract
Introduction: Xao Tam Phan (Paramignya trimera) has long been used in Viet Nam as an herbal medicine for the treatment of Hepatitis, hepatocellular carcinoma, and diabetes. This study aimed to determine the anti-proliferation effect of Paramignya trimera extract (P. trimera extract) on HepG2 hepatocellular carcinoma cells.
Methods: AlamarBlue assay was used to determine the IC50 values of P. trimera extract on HepG2 cells. Adipose-derived stem cells (ADSCs) was used as normal cell control. For apoptosis examination, P. trimera extract-treated HepG2 cells were incubated with Annexin V/Propidium iodide (PI). Then they have been analyzed their expression of Annexin-V and PI by flow cytometry. The cell nuclear degradation also was evaluated by PI/Hoechst 33342 staining assay.
Results: Doxorubicin and P. trimera extract IC50 values on HepG2 cells were 55.13 +/- 2.028 ng/ml and 582.533 +/- 16.521 mg/ml, respectively. Those on ADSCs were 5.96 +/- 0.56 ng/ml and 268.976 +/- 19.325 mg/ml, respectively. Side effect index value (SEI) of P. trimera extract was 2.175 +/- 0.12, and the SEI of doxorubicin was 8.71 +/- 0.36. Flow cytometry analysis indicated significant apoptosis on P. trimera extract-treated HepG2 cells at a dose of 500 mg/ml (32.39 +/- 2.28% apoptotic cells, and 14.63 +/- 1.59% necrotic cells). Nuclear aggregation and degradation was seen on 500 mg/ml P. trimera treated HepG2 cells.
Conclusion: P. trimera extract could inhibit HepG2 hepatocellular carcinoma cell proliferation by inducing apoptosis.
Introduction
According to Globcan 2018 statistics, 841.080 people were diagnosed with liver cancer worldwide; an estimated 781,631 people died from the disease1. Among the most common types of fatal cancer in the world, liver cancer has the fourth highest mortality rate and accounts for the second-highest death rate for men2. Among the regions, Asia is the most alarming region. Asia has consistently led the way in terms of new cases of diagnosis and mortality, as well as the proportion of people living with liver cancer. The highest is in West Asia and then Southeast Asia1.
According to IARC data in 2018, Viet Nam had 25335 new cases, 25404 deaths, and 21055 people living for five years with liver cancer3. Liver cancer accounts for the highest percentage of all common diseases, as well as the leading cause of death in Viet Nam. There have been many developed methods to treat cancer. Among them, five routine methods are commonly used: tumor removal surgery, chemotherapy, radiation therapy, hormone therapy, and immunotherapy4. Depending on the type of cancer, the patient's condition that the doctor chooses a suitable treatment or a combination of treatments to bring the best effect.
In particular, chemotherapy is still an important method to treat this disease. However, the cases of drug resistance are getting a problem. Besides, chemotherapy also causes many side effects, the possibility of cancer recurrence. Research on developing new drugs is still being promoted, including the use of compounds derived from nature, especially medicinal plants from ancient times. Up to date, folk remedies still play an essential role in the treatment and protection of human health. Vinca alkaloid, saponins, flavonoids, and their derivatives are anti-cancer compounds isolated from plants and scientifically proven to treat cancer7, 6, 5.
Vietnam is located in the tropics, with abundant biological resources, including many herbs that, according to folk medicine points, can cure cancer. However, most are used according to folk experience or used as a functional food, without precise mechanism.
In Vietnam and Thailand, extract is considered a folk medicine to treat hepatitis, liver cancer, and diabetes8. However, studies on in general and their medicinal properties are quite limited.
Xao Tam Phan is a small tree with a climbing body; body more than 4 m long, about 10 cm diameter; body and branches hairless, spiky, and to 7 to 8 cm long, slightly curved down. Leaves grow in a cluster or three-fold pattern; thick slab; oblong to extended narrow circles; size 10 – 18 x 1 – 2.5 cm; The whole cover is slightly bent; tendon filler 8 – 10 pairs; petioles short, 2 - 3 mm long. The parts of the plant have the essential oils, and their roots are at most8. In Vietnam, the tree grows mainly in tropical forests, especially in the central provinces such as Khanh Hoa, Ninh Thuan, and Binh Thuan, in addition to areas adjacent to Laos and Cambodia.
According to folk experience, roots are used as medicine. root has a slightly bitter taste, slightly acrid, slightly sweet. According to some analyzes, extract contains saponin (267.15 mg EE/g dry sample), proanthocyanidin (3.98 mg CE/g dry sample), phenolic (11.27 mg GAE/g dry sample), flavonoid (19.88 mg RE/g dry sample)9. Besides, extract has also been shown to contain coumarins with potent anti-inflammatory properties, including ostruthin and ninhvanin10. Moreover, there are also studies proving has many antioxidants. In another recent study, it was shown that extract has a strong ability to inhibit MCF-7 breast cancer cells in both 2D and 3D culture conditions11.
However, there have not been many studies on the anti-proliferation of this plant on HepG2 hepatocellular carcinoma cells. Therefore, this study aimed to assess the effects of extract on HepG2 hepatocellular carcinoma cells.
Material — Methods
Cell culture
HepG2 hepatocellular carcinoma cells were obtained from ATCC (Manassas, VA). They were cultured in DMEM/F12 medium (Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum (FBS) and 1% antibiotic-mycotic (Gibco, Thermo Fisher Scientific, MA). HepG2 cells were cultured at 5% COhumidified atmosphere at 37C. Cells were passed at 80% confluency by trypsin/EDTA (0.025%) (Gibco, Thermo Fisher Scientific, MA).
Adipose-derived stem cells (ADSCs) were isolated from adipose tissues with consent from the donor as the published protocol12. Adipose tissues were kept in cool temperature in PBS solution containing 1% antibiotic-antimycotic solution, and transferred to the laboratory for subsequent processing. The stromal vascular fraction (SVF) was extracted from the adipose tissue by the Cell Extraction Kit (Regenmedlab, Ho Chi Minh City, VN). Then SVF was cultured to collect ADSCs per a previously published protocol. All assays were performed per the published study12.
Cytotoxicity assay
HepG2 and ADSC cells were seeded in 96 wells plate. The cell density was 2500 cells per well. After 24h, cells were treated with P. extract at concentration 2000, 1000, 500, 250, 125, 60,30,15,7 µg/ml. Parallelly, HepG2 cells also treated with doxorubicin at concentrations of 2000, 1000, 500, 250, 125, 60, 30, 15,7 ng/ml. After 48h incubation, cells were evaluated viability by Alamar Blue assay.
Side Effect Index (SEI) is calculated using the formula:
SEI =
SEI =
Apoptosis assay
HepG2 cells were seeded in 6 wells plate. The cell density was 200.000 cells per well. Cells were treated with extract at concentrations of 60, 125, 250, 500, and 1000 µg/ml and incubated in 48h. Cells were labeled with 3 µL Annexin V-FITC (BD Biosciences, Franklin Lakes, NJ) (and 3 µl of Propodium Iodide (PI) in 500 µl of binding buffer for 15 mins to detect apoptotic and necrotic cell death using a FACSCalibur Flow Cytometer. Data were analyzed by Cellquest software (BD Biosciences).
Nuclei staining assay
HepG2 cells were added into a 6-well plate with a density of 200000 cells/well. HepG2 cells were treated with extract at concentrations of 2000 µg/ml, 500 µg/ml, 125 µg/ml, and control for 48 hours. Similarly, doxorubicin was added at concentrations of 300 ng/ml, 60 ng/ml, 15 ng/ml, and control for 48 hours. Then, cells mixed with 50 ml Hoechst 33342 and 10 ml Propidium Iodide, incubated for 10 minutes. Finally, the cell suspension was dropped in the lame, observed under the microscope fluorescence objective 40X (Carl-Zeiss, Germany).
Results
Cytotoxicity of extract and doxorubicin on HepG2 cells
The results showed that IC values of extract on ADSCs and HepG2 cells were 268.98 ± 19.33 µg/ml and 582.53 ± 16.52 µg/ml, respectively (Figure 1). The IC values of doxorubicin on ADSCs and HepG2 cells are 5.96 ± 0.56 ng/ml and 55.13 ± 2.028 ng/ml, respectively. We performed the side effects index (SEI) of and doxorubicin. SEI is considered as a method to assess the toxicity of a substance on a normal cell line. The result showed that SEI value of was 2.175 ± 0.12, and SEI value of doxorubicin was 8.71 ± 0.36.

Anti-proliferation of
Apoptosis of HepG2 cells after treatment of extract
Results showed that the total percentage of late apoptosis increased in dose-dependent manner. Late apoptosis population at concentrations of extract 60, 125, 250, 500, 1000 µg/ml increased by 6.687 ± 0.78%, 7.28 ± 0.85%, 9.77 ± 1.35%, 7.743 ± 2.67%, 32.82 ±2.28%, 49.28 ± 2.43% respectively (Figure 2). At the concentration of 500 µg/ml, the total cell death counted almost 50%. When the concentration increased up to 1000 µg/ml, cell death was at 80%. This result proves that extract can induce apoptosis in HepG2 cells.

Apoptosis population of HepG2 after treatment with
Cell nuclei fragmentation of HepG2 cells after treatment of and doxorubicin extracts
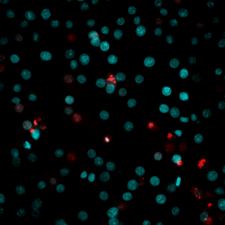
PI and Hoechst 33342 staining results showed in both . extract treatment group, and doxorubicin treatment group, the number of dead cells increased in the manner of concentration dependence. These cells have DNA compressed or fragmented. In control, cells were mainly in blue fluorescent with contact cell nuclei. When cells were treated with . extract at the concentration of ICvalue and at 1/4 of ICvalue, dead cells were significantly increased compared to that in control (582.533 ± 16.521 µg/ml) at IC, and 49.33 ± 2.50% at ¼ of IC compared to 7.00 ± 1.00% at control). Particularly, when cells were treated with . extract at 4 x IC concentration, most cells were no longer intact (94.00 ± 6.25%), stained with red fluorescent (Figure 3). This result proves that . extract can cause HepG2 cell death and nuclear fragmentation.

DNA fragmentation of HepG2 cells (magnification at X40). HepG2 cells were treated with
DISCUSSION
According to the World Cancer Research Organization (IARC), Vietnam is in an epidemiological area with a high rate of liver cancer. In 2018, liver cancer led to the number of new cases and deaths in the country. Studies looking for extracts or compounds to treat the disease are always promoted13. About 80% of cancer medicines approved by the US Food and Drug Administration (FDA) are derived from natural compounds, mainly from medicinal plants14. The rich and diverse biological resource of Vietnam is an abundant source of medicinal materials. There are many types of plants used as medicines. However, there is no scientific evidence of a transparent effect. Among them, Xao tam phan () is a plant belonging to the Citrus family (Rutaceae). In our previous study, extract showed a strong inhibitory effect on MCF-7 breast cancer cell lines in 2D culture conditions (260.8 ± 16.54 μg/ml) and 3D (IC 168.9 ± 11.65 μg/ml). It was shown that has a full inhibitory effect on the invasion of MCF-7 cells14. To clarify the impact of extract on liver carcinoma line, we conducted a test to determine the impact of this extract on HepG2 cells.
The IC value of extract was established on two lines of HepG2 cells and ADSCs, and doxorubicin used as a positive agent. The results showed that the average IC values of and doxorubicin on HepG2 cells were 582.53 ± 16.52 ng/ml and 55.13 ± 2.03 ng/ml. For ADSCs, . extract had an average IC value of 268.98 ± 19.33 µg/ml. The IC value of doxorubicin on ADSCs was 5.96 ± 0.56 ng/ml. Besides, the side effects index SEI values of and doxorubicin were 2.175 ± 0.12 and 8.71 ± 0.36. This result proves that in addition to the inhibitory effect on the MCF-7 cell line published in the previous study 11, extract also has anti-proliferation effects on the HepG2 cells and has less side effects than doxorubicin. This could be a promising potential for cancer treatment.
Doxorubicin is one of the most commonly used drugs in cancer chemotherapy. The study showed that doxorubicin was removed from the body by reducing two electrons by carbonyl reductase, which is most important in the liver15. Doxorubicin resistance is also due to over-expression of the adenosine triphosphate-binding cassette (ABC) family, typically ABCB1 (MDR1; MDR1) or ABCC1 (MDR1-related gene 1; MRP1)17, 16. These may be the cause of higher IC50 in HepG2 than those in ADSC.
Apoptosis is an important process for getting rid of cells that lose function in the body. Cancer cells, though not functioning, still exist due to their ability to evade programmatic death. The anticancer drug, therefore, aims to resist cell division or kill cancer cells through apoptosis or necrosis18. Flow cytometry result showed that after treatment of extract, apoptosis population cells increased by 6.687 ± 0.78%, 7.28 ± 0.85%, 9.77 ± 1.35%, 7.74 ± 2.67%, 32.82 ± 2.28%, 49.28 ± 2.43% correspond to concentrations of 60, 125, 250, 500 and 1000 µg/ml. In which the concentration of 500 µg/ml showed a significant increase in cyclic cell death rate (31%). This result showed that can activate the programmed pathway of death. Nuclear fragmentation, an important feature of programmed cell death, is biological damage at the molecular biology level. Results showed that cells treated with have nuclei compressed, divided into small pieces, the cell membrane remains intact. This result provides further evidence that extract has the ability to inhibit HepG2 cells through the programmed cell death.
CONCLUSION
Cancer is the leading cause of death worldwide. Live cancer is high rate of death in Vietnam. Traditional medicine plays the critical role in drug development. Vietnam possesses tropical forest with resources of potential herbal medicine. is popularly used in primarily treatment of some disease. In this study, has shown that it can anti-proliferate HepG2 cells at IC 268.98 ± 19.33 µg/ml, while has less side effect on ADSCs compared to doxorubicin. This implicated that is the potential herbal in further investigation of drug development for cancer.
Abbreviations
ADSC: adipose-derived stem cell
: Paramignya trimera
SEI: side effects index
Competing Interests
All authors equally contributed in this work. All authors read and approved the final version of the manuscript for submission.
Authors' Contributions
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by the Vietnam National University, Ho Chi Minh City, Vietnam, under grant A2015-18-01.

