Effects of pH, temperature and oxygen-limited condition on the virulence of Vibrio parahaemolyticus
- School of Biotechnology, International University, Vietnam National University of HCMC
Abstract
Introduction: Vibrio parahaemolyticus is a popular Gram-negative bacterium in the marine and estuarine regions. It can cause Early Mortality Syndrome (EMS), now named Acute Hepatopancreatic Necrosis Disease (AHPND), which resulted in severe losses to the shrimp culture. This study aimed to investigate the effect of pH, temperature, and oxygen-limited condition on the extracellular enzymatic activity of V. parahaemolyticus.
Methods: V. parahaemolyticus XN9, an AHPND-causing strain, was cultured in Brain Heart Infusion (BHI) medium at different pHs (7.5, 8.0, 8.5 and 9.0), temperatures (25oC, 30oC, and 35oC) and different oxygen conditions (either 120rpm shaking or static with the presence of oxygen absorber packages). The activity of five extracellular enzymes, including caseinase, lecithinase, chitinase, gelatinase, and lipase, was assessed using the agar-based method with the corresponding media.
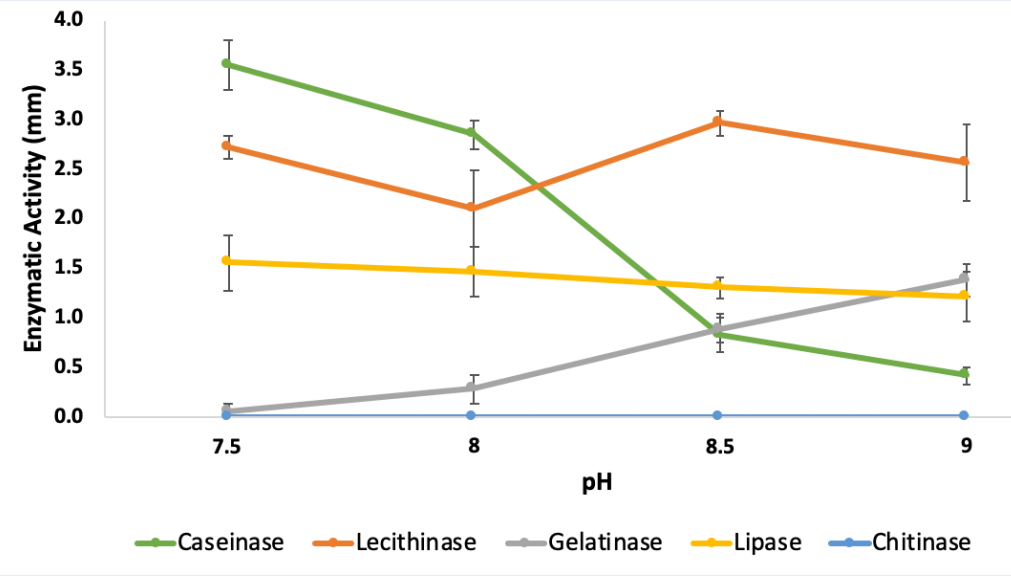
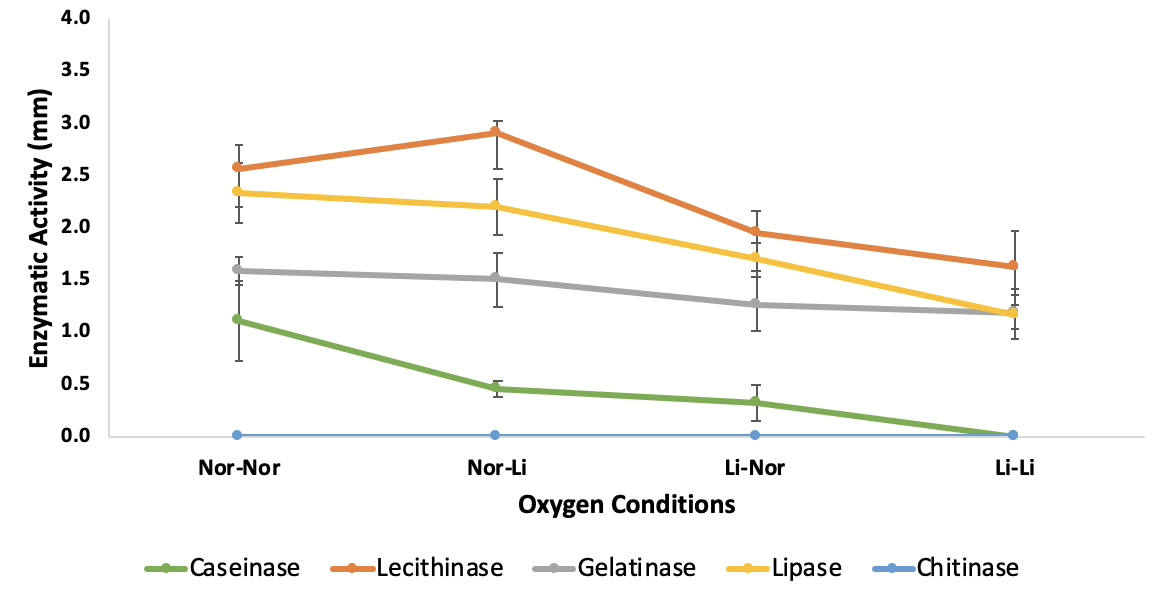
Results: When pH was increased from 7.5 to 9.0, caseinase and lipase activity was decreased significantly by 88% and 44%. In contrast, gelatinase activity increased markedly from 0 to 1.38 ± 0.17 (+) mm, and lecithinase reached the highest activity, which was 2.96 ± 0.13 mm (++) at pH 8.5. Regarding effect of temperature, highest activity of caseinase (0.85 ± 0.13 mm (+)) and gelatinase (1.37 ± 0.25 mm (+)) was obtained at 350C, lecithinase at 30oC and lipase at 25oC. Regarding the effect of oxygen level, the activity of most tested enzymes decreased significantly following the decrease of oxygen level. The highest activity of caseinase, gelatinase, and lipase was observed when the bacteria were cultured and tested in a fully oxygenated condition while lecithinase showed the highest activity when the bacteria were cultured in oxygenated condition but tested in oxygen-limited condition. No chitinase activity was observed in any of the tested conditions.
Conclusion: Our data suggested that extracellular enzymatic activity of V. parahaemolyticus is significantly influenced by environmental conditions. No particular testing condition resulted in the highest activity for all tested enzymes. However, warm temperature (30/ 35oC), mildly alkaline pH (pH 8.0), and fully oxygenated condition could increase the overall extracellular enzymatic activity of V. parahaemolyticus, thus increase its potential virulence.
Introduction
is a halophilic Gram-negative bacterium. It lives ubiquitously as a free-living organism in the marine environment or a colonizer of many different kinds of marine organisms 1. This motile, curved shaped bacterium, is a well-known causative agent of food-borne acute gastroenteritis in humans due to the consumption of raw or undercooked seafood 2, 3, 4. is also known to cause Early Mortality Syndrome (EMS) or Acute Hepatopancreatic Necrosis Disease (AHPND), which affects penaeid shrimp, causing massive death in larvae and young adults 5, 6. In recent years, AHPND has brought devastating effects to the shrimp industry of various countries such as China, Vietnam, Malaysia, Philippines, Thailand, and Mexico 7, 8, 9. AHPND pathogenesis is mainly caused by a binary toxin PirA/B encoded on a plasmid in 10. However, whether other toxins may also take part in causing this disease is still under investigation. The potential of a pathogen to cause so-called disease virulence reflects its ability to colonize, invade, escape the immune system, and obtain nutrition from the host. An important part of bacterial virulence is the ability to produce and secret extracellular enzymes to break down and digest nutrients from the environment 11, 12. However, the production of these enzymes is highly influenced by environmental factors such as nutrient supplement, dissolved oxygen, pH, temperature...13. In this study, the activity of five extracellular enzymes, including caseinase, lecithinase, gelatinase, lipase, and chitinase, was examined under the different pHs, temperatures and oxygen levels.
Materials- Methods
Bacteria strain
XN9, an AHPND-causing isolate, was kindly provided by Nha Trang University 14. It was streaked from glycerol stock on Thiosulfate-citrate-bile salts-sucrose agar (TCBS, Himedia, India). One colony was picked up for overnight culture in BHI medium at optimal culture condition (pH 8.5, 2.5% NaCl, 30C, and static condition) described previously (14). For assessing the effect of culture condition on the activity of extracellular enzymes, pH, temperature, and oxygen level were adjusted around the optimal culture condition (pH 8.5, 2.5% NaCl, 30C, and static condition). So, the testing conditions were 7.5, 8.0, 8.5 and 9.0 for pH; 25C, 30C and 35C for temperature, and 120 rpm shaking or static condition with the presence of Oxygen absorber package (O-Buster, Hsiao Sung, Non-Oxygen Chemical Co. Ltd., China) adhered to the bottom side of the falcon cap for oxygen level.
Extracellular enzymatic testing
Egg-yolk agar containing 1mL of Egg Yolk Emulsion (Himedia), Tributyrin Agar (Himedia) with added Tributyrin (Himedia) (10mL/L), BHI agar plates containing 1.5% (w/v) skim milk, 8% gelatin and 2% (w/v) colloidal chitin was used for lecithinase, lipase, caseinase, gelatinase, and chitinase, respectively. The colloidal chitin was prepared, as previously described 15. The overnight culture of V. parahaemolyticus was adjusted to OD600nm of 0.08- 0.1, then 10 µL of this bacterial suspension was dropped onto agar plates corresponding to the tested enzymes mentioned above. In the case of assessing the effect of oxygen, the overnight culture was dropped on the plate either without or with AnaeroPack® (Mitsubishi Gas Chemical, Japan) and plastic wrap. Positive controls used in these tests included ATCC29213 for caseinase and lipase, for lecithinase and gelatinase and for chitinase. After inoculation, the plates were incubated 24 hours for caseinase, gelatinase, and lipase and 48 hours for lecithinase and chitinase. For gelatinase, before reading the result, the agar plate was flooded with saturated ammonium sulfate ((NH)SO) to precipitate the undegraded gelatin. Clear halos surrounding the bacterial drop indicated the activity of the tested enzymes 16, 17, 18. All the tests were triplicated.
Data analysis
Enzyme activity (EA) was calculated using the formula: where D is the diameter of the bacterial drop plus the clear halo zone (mm), and dis the diameter of the bacterial drop itself (mm). It is graded (-) if there was no visible hydrolytic area; (+) if the EA value is less than 2 mm and (++) if equal or higher than 2 mm (15). Each test was triplicated, and the obtained data were analyzed using two-way ANOVA (Excel software, Microsoft 7) 14.
Results
Effects of pH on extracellular enzymatic activities of
Following the increase of pH from 7.5 to 9.0, caseinase activity decreased significantly by nearly 88% from 3.55 ± 0.25 (++) to 0.41 ± 0.08 mm (+) and lipase activity decreased by roughly 44%, from 1.83 ± 0.29 (+) to 1.21 ± 0.25 mm (+). In contrast, there was a significant increase of gelatinase activity from an undetectable level at pH 7.5 to 1.38 ± 0.17 mm (+) at pH 9.0. On the other hand, lecithinase activity of was recorded as strong (++), in all tested pHs with the highest value obtained at pH 8.5 (2.96 ± 0.13 mm). Chitinase activity was not observed in any tested pHs (Figure 1,
Effect of pH, temperature and oxygen level on the extracellular enzymatic activity of
| Standard: | pH | Temperature | Oxygen condition | |||||||
| pH 8.5/ 30oC | 7.5 | 8.0 | 9.0 | 25oC | 35oC | Nor-Nor | Nor-Li | Li-Nor | Li-Li | |
| Caseinase | 0.82 ± 0.17(+) | 3.55 ± 0.25(++) | 2.85 ± 0.14(++) | 0.41 ± 0.08 (+) | 0.67 ± 0.10 (+) | 0.85 ± 0.13 (+) | 1.10 ± 0.31(+) | 0.46 ± 0.06(+) | 0.33 ± 0.13(+) | _ |
| Lecithinase | 2.96 ± 0.13(++) | 2.72 ± 0.12(++) | 2.1 ± 0.39(++) | 2.57 ± 0.39(++) | 1.10 ± 0.15(+) | 1.87 ± 0.15(+) | 2.56 ± 0.19(++) | 2.92 ± 0.08(++) | 1.94 ± 0.18(+) | 1.62 ± 0.29(+) |
| Gelatinase | 0.89 ± 0.15(+) | _ | 0.28 ± 0.14(+) | 1.38 ± 0.17(+) | 0.86 ± 0.14(+) | 1.37 ± 0.25(+) | 1.59 ± 0.11(+) | 1.50 ± 0.21(+) | 1.27 ± 0.20(+) | 1.18 ± 0.13(+) |
| Lipase | 1.4 ± 0.1(+) | 1.83 ± 0.29(+) | 1.77 ± 0.25(+) | 1.21 ± 0.25(+) | 2.67 ± 0.22(++) | 2.25 ± 0.23(++) | 2.33 ± 0.23(++) | 2.20 ± 0.22(++) | 1.69 ± 0.14(+) | 1.17± 0.20(+) |
| Chitinase | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
Two-way ANOVA in analyzing the effect of A) four pH levels (7.5, 8.0, 8.5 and 9.0); B) three temperatures (25, 30 and 35oC); C) four tested oxygen conditions (Nor-Nor; Nor-Li; Li-Nor and Nor-Li) on the enzymatic activities of
| A) | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Sample | 50.3623567 | 4 | 12.5905892 | 335.719203 | 3.4326E-30 | 2.60597495 |
| Columns | 2.31145833 | 3 | 0.77048611 | 20.5444701 | 3.2315E-08 | 2.8387454 |
| Interaction | 23.97035 | 12 | 1.99752917 | 53.26271 | 1.0043E-20 | 2.0034594 |
| Within | 1.50013333 | 40 | 0.03750333 | |||
| Total | 78.1442983 | 59 | ||||
| B) | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Sample | 27.6018133 | 4 | 6.90045333 | 324.982104 | 3.1206E-24 | 2.68962757 |
| Columns | 0.35015111 | 2 | 0.17507556 | 8.24531659 | 0.00140067 | 3.3158295 |
| Interaction | 7.94242667 | 8 | 0.99280333 | 46.7568289 | 7.5498E-15 | 2.26616327 |
| Within | 0.637 | 30 | 0.02123333 | |||
| Total | 36.5313911 | 44 | ||||
| C) | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Sample | 42.5853233 | 4 | 10.6463308 | 253.032224 | 8.0542E-28 | 2.60597495 |
| Columns | 5.012045 | 3 | 1.67068167 | 39.7072292 | 4.5515E-12 | 2.8387454 |
| Interaction | 2.85693 | 12 | 0.2380775 | 5.65840761 | 1.4569E-05 | 2.0034594 |
| Within | 1.683 | 40 | 0.042075 | |||
| Total | 52.1372983 | 59 |

Extracellular enzymatic activity of
Effects of temperature on extracellular enzymatic activities of
The rise of temperature from 25C to 35C led to strong decomposition of gelatin in the BHI medium with EA value increased by 56% from 0.86 ± 0.14 (+) to 1.37 ± 0.25 mm (+). It also resulted in slight increase of caseinase from 0.67 ± 0.10 (+) to 0.85 ± 0.13 mm (+). At 30C, the obtained EA value was highest for lecithinase (2.96 ± 0.13 mm (++)) but lowest for lipase (1.4 ± 0.1 mm (+)). Chitinase activity was again not observed in any tested temperatures (Figure 2,

Extracellular enzymatic activity of
Investigating the effects of oxygen on extracellular enzymatic activities of
Under the limited oxygen presence, most of the tested enzyme activities were low or even not observed. Caseinase activity declined considerably from 1.10 ± 0.31 mm (+) in the case of both fully oxygenated culture and testing to 0 mm (-) in case of both limited oxygen culture and testing. Similar trend was seen in case of lipase and gelatinase, with enzyme activity decreased markedly about 49% from 2.33± 0.23 mm (++) to 1.17± 0.20 mm (+) and 25% from 1.59 ± 0.11 (+) to 1.18 ± 0.13 mm (+) respectively. In case of lecithinase, this enzyme activity expressed in most tested conditions with lowest activity (1.62 ± 0.29 mm (+)) in case of both limited oxygen culture and testing and highest activity (2.92 ± 0.08 mm (++)) in case of oxygenated overnight culture followed by limited oxygen testing condition. No activity of chitinase was observed in any case (Figure 3,
Extracellular enzymatic activity of
Discussion
Our data indicated that the production of extracellular enzymes in was highly affected by environmental factors. In inappropriate conditions, the production of some enzymes can be minimized to undetected levels such as gelatinase in case of pH 7.5 or caseinase in case of limited oxygen condition. On the other hand, some enzymes, such as lecithinase seemed to be constantly and strongly produced in most conditions tested in our study. The strong production of lecithinase was observed not only in but also in other Vibrio species 17, 19, 20, 21. We did not detect chitinase activity in any tested conditions. Chitinase is a typical virulence factor of marine bacteria that can breakdown glycosidic bonds in the chitin of shrimp and other marine organisms 22. The absence of chitinase activity indicated that this AHPND strain may not utilize chitinase attack aquatic crustaceans or chitinase might not be induced
Regarding pH, our data showed that while most of the tested enzymes showed the highest activity at pH 7.0, gelatinase only expressed its activity at alkaline conditions. This is in agreement with previous studies showing that had a high rate of hydrolysis of gelatin in alkaline environments 23, 24. Gelatinase, together with lecithinase and protease, are constantly expressed in most disease-causing species, particularly strains 21, 25.
Temperature is a well-known factor that affects the growth of . It was shown that the minimal growth temperature of was 13C, and its optimal growth temperature was 30C 14, 26. However, for extracellular enzyme production, the optimal temperature was varied for different types of enzymes. Gelatinase, for examples was found to express the highest activity in species at 24°C 23. In our study, the optimal temperature was 35°C for caseinase, gelatinase and lipase, and 30°C for lecithinase.
like other species, are facultative anaerobe. Its growth is only hindered by strict anaerobic conditions but not limited oxygen condition. Some environmental study even showed that the number of in low oxygen marine water was higher than in high oxygen samples 27. Our data showed a decrease in the activity of extracellular enzymes for all tested enzymes, of which caseinase was the most affected one. No activity of caseinase was found when was cultured and tested in limited oxygen conditions. It was in agreement with a previous study showing that the production of proteolytic enzymes was negatively affected by a low dissolved oxygen level 28.
Conclusion
pH, temperature, and oxygen condition are essential factors affecting not only the growth of as previously shown(14) but also its extracellular enzyme activity. No culturing condition resulted in the highest activity for all extracellular enzymes was found. However, warm temperature (30/ 35C), mildly alkaline pH (pH 8.0), and fully oxygenated condition could increase the overall extracellular enzymatic activity of thus increase its potential virulence.
LIST OF ABBREVIATIONS
:
AHPND: Acute Hepatopancreatic Necrosis Disease
BHI: Brain Heart Infusion
EA: Enzyme activity
EMS: Early Mortality Syndrome
TCBS: Thiosulfate-citrate-bile salts-sucrose agar
COMPETING INTERESTS
The author(s) declare that they have no competing interests
ACKNOWLEDGEMENTS
We would like to thank Dr Nguyen Van Duy who generously provided the AHPND isolate for our study.

