Preparation of Heterogeneous Fenton-Type Nano Catalysts and Their Application to Methylene Blue Degradation
- University of Science, Ho Chi Minh City, Vietnam
Abstract
Introduction: Iron-based nanocatalysts are known as a new generation heterogeneous Fenton catalyst, replacing the traditional Fenton catalyst system which has many disadvantages in experimental processes and industrial applications. In this study, we focused on the preparation of iron nanoparticles and their use when embedded in traditional supports, as well as tested their catalytic activity by modified Fenton-type oxidation of methylene blue (MB) substrate.
Method: Scanning Electron Microscope (SEM), Transmission Electron Microscopy (TEM), X-ray diffraction (XRD) and UV-vis were used for physio-chemical characterization of the catalysts.
Results: Iron nanoparticles were obtained in the reduction of iron salt by sodium borohydride (NaBH4), with particle size in the range of 4-5 nm. Fe-X (X represents C, Bentonite, Al2O3, or ZnO) was synthesized in high yield and applied to the Fenton oxidation of MB; approximately 99% conversion was observed in the case of Fe-C.
Conclusion: Supported iron nanoparticles are active catalysts for the oxidation of MB; however, there are limitations if pH is above 3.
INTRODUCTION
In recent years, wastewater containing synthetic dyes have garnered increased attention due to factors such as continuous discharge, low toxicity and biodegradation1, 2. Specifically, the oxidation of methylene blue (MB) and basic dyes of thiazine are receiving greater interest. Therefore, treatment by Fenton method has been attracting many researchers3, 4, 5, 6.
Iron-based nanocatalysts are known as a new generation heterogeneous Fenton catalyst which can replace the traditional Fenton catalyst system that has many disadvantages in experimental processes as well as in industrial applications7. Both heterogenous and homogeneous Fenton catalysts show similar activity for the same amount of catalyst used. However, in the case of traditional Fenton catalysts, there is a need for a considerable amount of chemicals and manpower to remove the mixture containing the iron catalysts8. Therefore, in order to improve the Fenton process, a number of reports have used supported iron catalysts for applications in drug sciences9, treatment of heavy metal pollution10, chemical catalysis11, and industrial textile and dyes12.
Moreover, due to the reduction of metal, ethylene glycol (EG) reduction is a common process for the preparation of metal nanoparticles. In particular, with iron reduction, high temperature or microwave irradiation is usually required to improve the reduction performance13, 14. Sodium borohydride (NaBH) is considered as a force reducing agent which can reduce ionic precursors at room temperature; a disadvantage of the NaBH process, however, is the formation of irregular particle size15. Consequently, the combination of the EG and NaBHprocesses can yield better reduction in the preparation of the nanoparticles.
In this study, we describe the preparation of iron nanoparticles and their use as a new catalyst and impregnated into traditional supports, as well as evaluate their catalytic activities by modified Fenton-type oxidation of MB substrate.
MATERIALS - METHODS
Materials
Unless otherwise noted, all procedures were carried out in air. Reagent grade iron(II) sulfate heptahydrate 99.5% (FeSO.7HO), ethylene glycol 99.5% (EG), methylene blue (MB), and sodium borohydride 98% (NaBH) were purchased from Merck (Germany). Aluminum oxide (AlO), zinc oxide (ZnO), hydrogen peroxide (HO), and polyvinyl pyrrolidone-K30 (PVP) were purchased from various Chinese suppliers (Xilong, China). Binh Thuan bentonite (Bent) and activated carbon were purchased from local suppliers (Binh Thuan, Vietnam). Absolute ethanol and methanol were supplied by CHEMSOL (Ho Chi Minh city, Vietnam).
Characterization
The morphology of iron catalysts was examined by scanning electron microscope (SEM) (Hitachi S4800, Japan). Transmission Electron Microscopy (TEM) images was collected using FEI Tecnai G2 F20 (University of Technology, Ho Chi Minh city). The X-ray diffraction (XRD) data of all samples were collected in a Philip X-Ray (Vietnam Petroleum Institute, Ho Chi Minh city) with Cu K radiation running at 35 kV/30 mA in the 2θ range 5°-75° with a step size of 0.2°/min. Nitrogen adsorption–desorption isotherms were collected at 77K using Brunauer–Emmett–Teller calculation (BET, AUTOSORB-1C Quantachrome); all samples were degassed at 100 °C and 10 Pa. UV spectras were recorded on Agilent Cary 60 UV-Vis (Applied Physical Chemistry Laboratory of VNUHCM-University of Science, Ho Chi Minh city). Atomic Absorption pectroscopy (AAS) was analysed on Agilent 240AAS (Laboratory of Analysis-University of Science, Ho Chi Minh city). All the catalytic test were performed in a Multireactors Carousel 12 lus system (Laboratory of Catalysis-University of Science, Ho chi Minh city).
Catalyst preparation
To a two-necked roundbottom flask, FeSO.7HO (0.5 g, 1.8 mmol) and 50 mL of deionized (DI) water were added. After stirring for 15 min, 25 mL of absolute ethanol and 1 g of PVP were added into the mixture. In another flask, a mixture of EG (2.0 mL) and NaBH (0.1 g, 2.7 mmol) in 50 mL of DI water were prepared. Then, the reducing agent solution was added in a dropwise fashion to the mixture of iron salt, which was stirred until the solution appeared black.
The iron nanoparticles were then loaded on the supports (X), such as AlO, bentonite, ZnO or activated carbon, under vacuum at room temperature. The process was repeated several times to make sure all the iron nanoparticles were loaded onto X.
Catalyst evaluation
In this study, the activity of the catalyst was investigated via the degradation of MB under aqueous solution. The catalytic evaluation of Fe-X was carried out in a 20 mL multireactor with stirring at room temperature. In this process, 5.0 mol% of Fe-X was used in the MB aqueous solution of 40 mg/L (10 mL), and 1.0 mL of 30% hydrogen peroxide solution in water. The influence of pH on the process (at pH 3.0, 5.0, and 7.0) was observed by dosing hydrochloric acid. The concentration changes of MB were recorded by the colorimetric method at the maximum absorbance of 664 nm. Reproducibility was checked by repeating the measurement several times and was found to be within acceptable limits.
RESULTS
Iron nanoparticles were prepared by the reduction of FeSO.7HO using a combination of EG and NaBH as reducing agent. The original blue solution turned dark when Fe nanoparticles formed. The solution was then subjected to UV-Vis measurement.

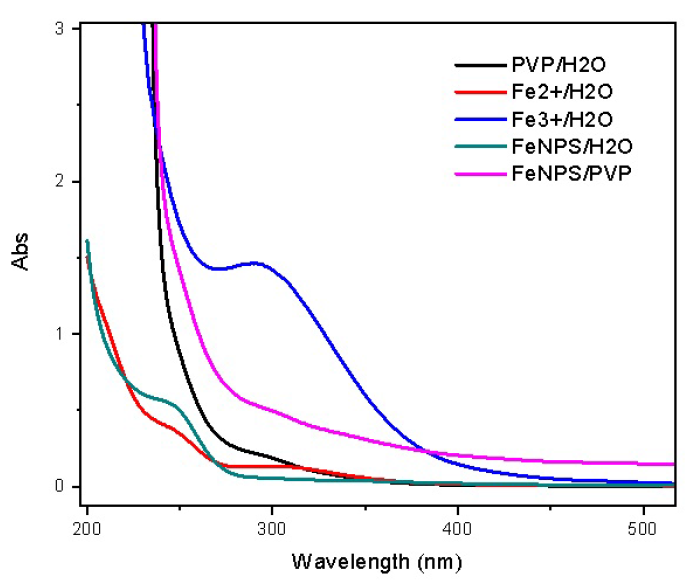
UV-vis absorbance spectra of the solution of iron nanoparticles which were reduced by a combination of EG and NaBH4 at room temperature.
Figure 1 show the UV-Vis spectra of the iron nanoparticles prepared at room temperature. The absorption range of 300-320 nm was assigned for the absorption peaks of Fe and Fe ions. The solution of PVP did not absorb at the UV zone.
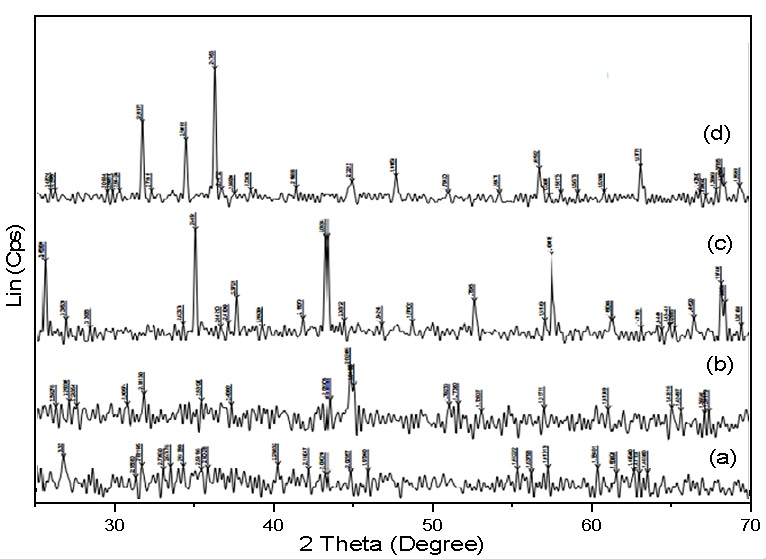
XRD patterns of: A) Fe-Bentonite, B)Fe-C, C) Fe-Al2O3, and D) Fe-ZnO; all were dried at 60 °C under vacuum for 8 h.
In addition, the solution of iron nanoparticles were further analyzed by TEM technique. TEM images were taken at 20 nm, 50 nm, and 100 nm. As illustrated in Figure 3, the average size of the iron nanoparticles was in the range of 4-5 nm.
According to AAS analysis, the concentration of supported iron nanoparticles as Fe-AlO, Fe-ZnO, Fe-Bent, and Fe-C were recorded at 14.20, 13.95, 12.00, and 10.77 wt%, respectively.
The surface morphology of Fe-X was characterized by SEM. As illustrated in Figure 4, the surface of Fe-AlO (A) showed uniform particles, with a number of spherical shape formed between the pores. Likewise, the surface of Fe-Bentonite (D) showed a similar morphology; both samples had similar specific surface area. In contrast, in the case of Fe-C (B), from the thickness of the slit-shaped pores which formed around the surface, it appeared that most of iron nanoparticles had not successfully attached to the activated carbon. Unfortunately, in the case of Fe-Bent (C), the surface was smooth, with a few pores, and with big cubic shapes covering the surface of the catalyst. It is worth noting that during the loading process, the spherical shape could be destroyed.

TEM images of Fe nanoparticles taken at 20, 50, and 100 nm.
Moreover,
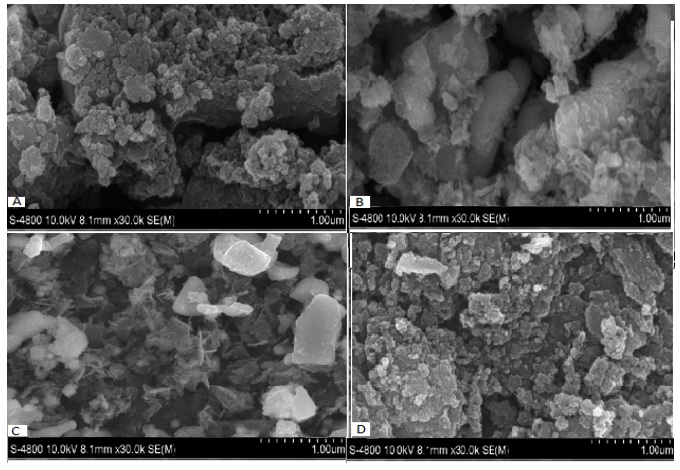
SEM images of: A) Fe-Al2O3, B) Fe-C, C) Fe-ZnO, and D) Fe-Bent. The images were taken at 1.0
In order to evaluate the oxidation activity of the catalysts, 5.0 mol% of the Fe-X catalysts was used to oxidate MB solution in the presence of hydrogen peroxide. All experiments and results are summarized in Figure 5.

Catalytic activities of Fe-X over MB conversion. Reaction condition: 5 mol% of catalyst at pH 3, pH 5, and pH 7; [MB] = 40 mg/L; reaction time was 60 min.
DISCUSSION
According to H. Chen16, the characteristic spectra of iron nanoparticles are at two peaks, λ = 216 nm and 268 nm. In Figure 1, an absorption peak at 268 nm was observed in the solution of iron nanoparticles, even if it was slightly smooth and seemed to be obscured. In addition, there was no absorption peaks of the Fe and Fe ions in this solution. This demonstrates that almost all Fe ion were successfully reduced to Fe nanoparticles. This was also confirmed by XRD pattern in Figure 2, in which four cases showed the characteristic peak at 2 angles of 44.9° (assigned for metallic iron), even though the peaks were rather weak due to the low concentration of iron nanoparticles in the samples.
Specific surface areas of catalysts
|
Catalysts |
SBET (m2.g-1) |
|
Fe-Al2O3 |
69.98 |
|
Fe-C |
224.69 |
|
Fe-ZnO |
27.78 |
|
Fe-Bentonite |
41.81 |
As observed in Figure 3, the shape and size of the iron nanoparticles were determined to be spherical and in the range of 4-5 nm. These observations could be explained by the fact that the iron nanoparticles were protected and dispersed by the PVP molecules. Furthermore, the PVP molecules may have prevented the agglutination and deposition of the iron nanoparticles.
The catalytic oxidation exhibited an excellent decomposition of MB, as shown in Figure 5. All the iron-supported samples exhibited high activities, in particular Fe-C, which had the highest surface area and showed the best conversion (up to 99.7% in the case of pH 3). On the other hand, the influence of pH was also observed. For example, the conversion of MB was decreased when pH was increased to neutral. In the case of the Fe-ZnO catalyst, the conversion was decreased to 84.8% at pH 7, whereas it increased to 90.2% and 97.9% conversion at pH 5 and pH 3, respectively. It could be explained that in acidic environment , iron nanoparticles are more easily oxidized to iron ions, which play an important role in the Fenton-type heterogenous catalysis17. Likewise, in the case of other samples, the MB conversion decreased to 89.2% and 78.9% at pH 7, as was the case with Fe-C and Fe-Bent catalysts, respectively. However, it was still lower than that at pH 5 for the conversion of 95.6% (Fe-C) and 86.7% (Fe-Bent). It is clear that in the case of pH 5, a moderate conversion were obtained. In order to propose the specific mechanism (
CONCLUSION
This study prepared and characterized Fe-X particles (X = C, Bentonite, AlO, or ZnO) as catalysts. All the physio-chemical charaterization of the catalysts were evaluated in detail. All the results corroborated with the loading process. Indeed, XRD and TEM analyses indicated that iron was incoporated as Fe inside X. Moreover, the catalytic test indicated that almost all the supported iron nanoparticles exhibited high catalytic activities, especially at pH 3 where the conversion of MB reached 99.7% with the Fe-C catalyst.
ABBREVIATIONS
Bent: bentonites
DI: deionized
EG: ethylene glycol
MB: methylene blue
PVP: polyvinyl pyrrolidone-K30
Zeolit: zeolites
COMPETING INTERESTS
The authors declare that there are no conflicts of interest regarding the publication of this paper.
AUTHOR’S CONTRIBUTION
Co Thanh Thien has conceived of the present idea, carried out and written the manuscript. Le Dinh Khoi, Le Van De and Doan Thi Nhu Thuy carried out the experiments and analyzed all the samples.
ACKNOWLEDGMENT
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number C2019-18-13.

