Photocatalytic activity enhancement for removal of dye molecules based on plasmonic Ag grafted TiO2 nanocubes under visible light driven
- Faculty of Physics and Engineering Physics, VNUHCM-University of Science
- Faculty of Physics, Dong Thap University
- Faculty of Chemistry, VNUHCM-University of Science, Viet Nam
- Faculty of Physics and Engineering Physics, VNUHCM-University of Science, Viet Nam
Abstract
Introduction: Finding a novel photocatalyst for photocatalytic degradation operating in the wavelength range from UV to visible light has been considered a great potential for environmental remediation. Herein, TiO2 nanocubics (NCs) decorated Ag nanoparticles (NPs) with various concentrations were developed.
Methods: The crystal structure, morphological and chemical characteristics of prepared photocatalysts were thoroughly analyzed by a series of main analyses (X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDX), and UVVis spectra).
Results: The results revealed that a significantly promoting visible-light photocatalytic behavior of TiO2NCs@Ag photocatalyst was observed. The photocatalytic methyl orange (MO) degradation of the as-synthesized Ag anchored TiO2NCs photocatalyst (85% and 62% under UV light and visible light, respectively) exhibited outstanding photocatalytic efficacy compared with pristine TiO2 NCs. The achieved results could be assigned to the synergistic effects between TiO2NCs and Ag- NPs, leading to enhanced charge carrier separation and improved absorption ability in visible-light response.
Conclusion: This work facilitates designing and developing high-efficiency heterostructure photocatalysts for practical works related to environmental deterioration.
INTRODUCTION
The polluted environment caused by aromatic sulfur-containing compounds and organic dyes has become one of the most urgent issues in recent years 1, 2, 3. Therefore, the disintegration of poisonous organic for environmental purification based on green technologies and energy-efficient has attracted enormous attention. Recently, photocatalysis regarded as one of the advanced green technologies for environmental purification with zero harmful emissions and without additional pollutant emission has become one of the hot topics in the field of environmental remediation practice with the aid of light 4, 5, 6. Among these various semiconductor materials, TiO has been proved to be a promising candidate because of its chemical and biological inertness, high photo corrosion resistance, low cost, and environmentally friendly 7, 8. However, the photocatalytic performance of TiO has faced with two main obstacles: i) TiO with a large bandgap of 3.2 eV can only harvest under ultraviolet (UV) light photons, which constitutes a small fraction of total solar energy; ii) the high recombination rate of photogenerated electron-hole pairs resulting in decrease the photocatalytic productivity 9, 10. Hence, to address the above problems, many attempts have been proceeded to enhance the TiO photocatalytic performance, including cocatalyst decoration, doping bandgap engineering, the combination with other semiconductors and morphology control nanostructure construction, and morphology control 11, 12, 13. Among these approaches, sensitizing the surface through combining TiO with plasmonic metal nanoparticles to create heterostructure engineering has been a prominent area of scientific interest in recent years as the presence of plasmonic nanoparticles on the surface of these metal oxides that could provide several advantages. First, the noble metal incorporated with TiO may extend the absorption efficiency toward the visible light region through localized surface plasmon resonance (LSPR) 14, 15. A second outstanding advantage of functionalizing TiO with plasmonic nanoparticles was the improvement of photoinduced electron-hole pairs separation based on the formation of heterojunction related to the Schottky barrier at the metal−semiconductor interface, contributing efficient spatial charge separation 16, 17. For example, as reported by Gong et al., the combination of plasmonic noble metals (such as Au and Ag) with semiconductor would be a more promising option for photocatalytic activity due to the enhancement of absorbance in the visible regime and trapping the photogenerated charge carriers 18. As reported by Jafari et al., loading silver nanoparticles on the surface of TiO nanoparticles exhibited a higher RhB photocatalytic degradation compared with pristine TiO under UV light irradiation 19. Yin et al. reported that mesoporous TiO hollow shells exhibited a good photocatalytic behavior for the degradation of organic dye molecules 20. Yang et al. showcased that hollow TiO hierarchical boxes with appropriate anatase and rutile ratios showed a high light conversion ability 21. The plasmonic materials less than 10 nm could enable hot carrier formation. An optimal sizes in the range of 40-50 nm, they could harvest light efficiently 22. As a matter of fact, the morphology of noble metal-TiO could vitally affect the plasmonic resonance and their photocatalytic activity. Hence, based on the above discussion, a design of TiO nanomaterials with cubic structure with an enhancement light absorption capacity based on their high specific area was proposed. Moreover, the enhancement of TiO photocatalytic behavior in the visible regime by decorating the surface of TiO with spherical Ag nanoparticles was evaluated. The Ag-anchored onto TiO photocatalysts were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), ultraviolet-visible (UV-Vis) diffuse reflectance spectroscopy, and energy-dispersive X-ray (EDX). The improved performance of TiONCs@Ag was also proven in the photodegradation of methyl orange (MO) under visible light irradiation. A possible photocatalytic mechanism was projected based on the evaluation of photogenerated electron-hole pairs separation in photocatalytic activity.
EXPERIMENT
Materials
Titanium butoxide (Ti(CHO), Aldrich Chemical, <99%), tetramethylammonium hydroxide (CHNO, Merck), hydrochloric acid (HCl, Merck, <37%), and methanol (CHOH, Merck, <99.9%), silver nitrate (AgNO, > 99%, Merck), methyl orange (Merck, MO) and polyvinylpyrrolidone (PVP) were received and utilized for experiments without further purification. Double-distilled water was used during the experiments to prepare the required solutions.
Fabrication of TiO nanocubes (NCs)
The TiONCs were fabricated via the hydrothermal method. In a typical experiment, titanium butoxide (0.05 mol) was dissolved in double-distilled water (30 mL) and stirred at 50 °C for 1 h, followed by adding the tetramethylammonium hydroxide (0.017 mol) into the above solution at 0 °C. The resulting mixture was heated at 135 °C for five h. Finally, the mixed solution was transferred to a Teflon lined autoclave and heated at 230 °C for five h. The obtained precipitate was centrifuged and washed several times with water and ethanol aqueous solution, followed by drying under vacuum.
Preparation of Ag modified onto TiONCs (TiONCs@Ag)
Ag modified onto TiO using the photo-reduction method under UV light irradiation. Ag was also deposited on the surface of TiONCs via a 0.5 M AgNO salt solution as the Ag precursor. Firstly, 0.1 g TiONCs was added to 100 ml of an aqueous solution of AgNO with various concentrations of powder (the wt.% of Ag in the solution was 0.5, 1.0, and 1.5). Then, the suspension was vigorously stirred for 2 hours under UV light irradiation. Finally, the as-obtained black-colored products were centrifuged to separate the powder and washed with double-distilled water several times, and dried for 6h at 60 °C under vacuum.
Characterization
The characteristic crystallinity and the morphological topography of as-prepared products were characterized using X-ray diffraction analysis using Cu Kα radiation (λ=1.5406 Å) and field-emission scanning electron microscopy (FESEM, Hitachi S-4800) equipped with an energy dispersive Xray spectrometer (EDX) to determine the constituent elements. The UV-Visible absorbance spectra were measured on a UVVis-NIR Spectrophotometer (SHIMADZU UV-3600) from 200 to 800 nm at a scan rate of nm/min. Raman scattering spectra of the photocatalysts were evaluated by a Horiba XploRA PLUS Raman System using a 532 nm laser with a power 25W as the excitation source.
The photocatalytic activity of as-synthesized samples was monitored by photodisintegration of methylene orange (MO) dyes under the illumination of UV light and visible light over the time period of 150 min. Prior to light irradiation, a mixture of organic dye and photocatalyst were placed in dark for 30 min to establish an adsorption/desorption equilibrium state. The photocatalytic performance of photocatalyst was investigated by the change of adsorption intensity of MO aqueous solution as a function of illumination time at the wavelength of 460 nm using a UV-vis spectrophotometer (JASCOV670). The blank experiment was also done under the same experimental procedures. The % disintegration performance of MO dye was estimated by using the following equation:
MO disintegration performance (%) = [(C-C)/C]/100, where C and C have corresponded to the MO concentration at the initial absorbance and after illumination for time “t”.
RESULTS
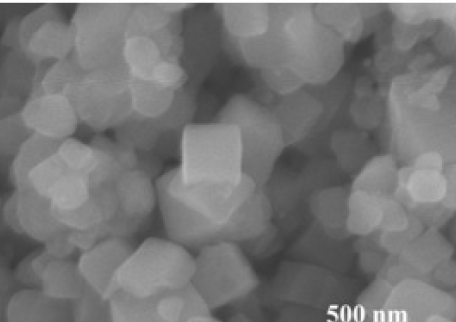
Figure 1 show the characteristic morphology of the as synthesized TiONCs and TiONCs@Ag. It indicated that the cubic TiO particles had well-shaped nanocubes and uniform size distribution with sizes of ca. 800 nm. Almost mono-dispersed structures of TiONCs could be observed in Figure 1(a). The SEM image (Figure 1(b)) revealed the uniform morphology of the as-prepared Ag NPs decorated onto TiONCs. As can be seen in Figure 1(b), the Ag NPs had spherical in shape on the surface of the TiONCs with an average diameter of ∼30 nm. The dispersive energy X-ray (EDX) spectra were used to collect the compositions of the photocatalyst, as depicted in Figure 1. It could be seen clearly that the EDX analysis (Figure 1(c-e)) proved the existence of Ti, O, and Ag, and no other impurities in the EDX analysis were observed. Moreover, Figure 1(f) provided evidence related to the corresponding elemental mapping for Ti, O, and Ag, indicating Ag was successfully attached to the surface of TiO NCs.

The morphological characteristics and chemical elements of the prepared photocatalyst. (a,b) SEM images of TiO2NCs and Ag grafted TiO2NCs, respectively. (c-e) elemental mapping of O, Ti, and Ag, respectively. (f) EDX spectrum of TiO2NCs@Ag heterostructures.
The crystal structure and phase confirmation of the as-synthesized TiO and TiONCs@Ag specimens were characterized by XRD patterns, as shown in Figure 2. The diffraction peaks of TiO located at 2 = 25°, 36°,41°,48°, 54.5°, and 57° corresponding to the reflection planes of (101), (103), (210), (200), (105), and (201) (JCPDS No. 21-1272), respectively, and could be attributed to the tetragonal anatase phase of TiO. No obvious peaks related to AgNPs were observed in the XRD patterns of TiONCs@Ag, which may be due to the low loading content of the metal on the surface of TiONCs. Moreover, the addition of AgNPs did not change the characteristic diffraction peaks of tetragonal anatase TiONCs. This demonstrated that the AgNPs only deposited on the surface of TiO without inserting into host structure.
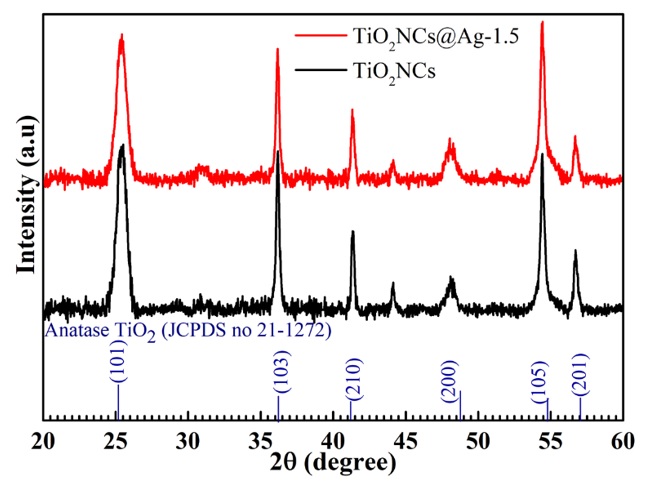
The crystal structures using XRD patterns of TiO2NCs and TiO2NCs@Ag photocatalyst.
To further ascertain the optical properties and the band gaps of as-prepared photocatalysts, UV−vis diffusion reflectance spectra of TiONCs and TiO NCs@Ag was characterized as shown in Figure 3. It showed that the plurality absorption of pristine anatase TiO possessing a wavelength region less than 400 nm (Figure 3a) with a bandgap of 3.3 eV was observed due to its large bandgap associated with a charge transfer from the valence band (VB) to the conduction band (CB), whereas, compared to pure TiO, Ag decorated TiO specimens exhibited a strong visible light absorption toward longer wavelengths corresponding to the bandgap of 3.1 eV (Figure 3b) which was derived from the localized surface plasmon resonance (LSPR) between the Ag nanoparticles anchored on the TiONCs surfaces. As the LSPR of Ag nanoparticles on the surface of TiONCs was excited by visible light related to the collective oscillation of electrons in the noble metal nanoparticles. Therefore, the photocatalytic performance of TiO could be significantly enhanced in the visible light.

The optical properties of photocatalyst through the UV-Vis absorption spectra (a), and plot of
Raman spectra were conducted to investigate the vibration modes, phase purity, and crystallinity of pristine TiONCs and Ag-modified TiONCs as shown in Figure 4. It was observed that The Raman spectrum of TiONCs located at 143.32, 202.61, 397.92, 514.89, and 635.22 cm was due to the presence of anatase phase TiO23, indicating that anatase nanoparticles were the dominant species. No signals associated to metal particles were recorded for the samples owing to the relatively low concentration of Ag grafted onto TiO. Moreover, the intensities of Raman peaks boosted with the decoration Ag NPs, and the position of the characteristic Raman peak of TiO has remained. This result showed that the modification of AgNPs onto TiONCs surface did not significantly change any phase transition and vibrational modes; however, it could cause a fluctuation of the electronic environment in the surroundings at the interface between TiO and Ag NPs 24, 25.
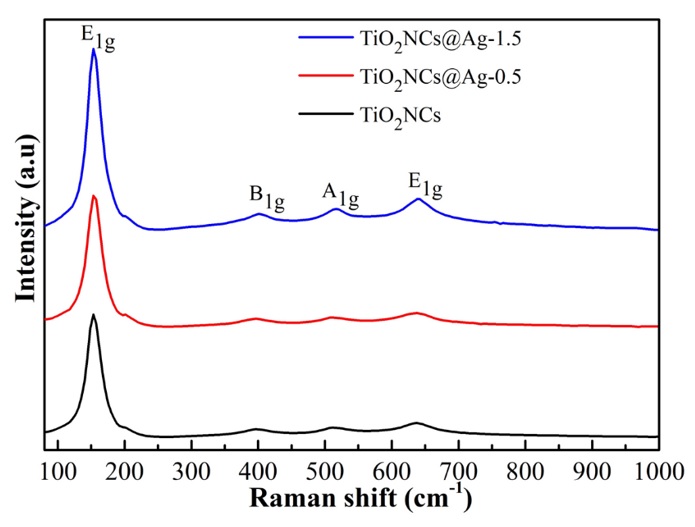
The vibration modes through Raman spectra of TiO2NCs and TiO2NCs@Ag specimens.
In order to elucidate the photocatalytic performance of pristine TiONCs and TiONCs@Ag photocatalyst, all as-prepared samples in this work were assessed under photodegradation of methyl orange (MO) as a model pollutant in the presence of UV light and visible after 150 min irradiation as shown in Figure 5. It could then be clearly observed that no MO aqueous solution photodegradation without the presence of any photocatalyst was recorded when irradiated under UV light and visible light illumination, whereas the degradation efficiencies for MO of TiONCs and TiONCs@Ag was remarkably changed, thereby confirming the efficiency of the photocatalyst. This could be explained by the higher charge separation depend on the generation of junctions between TiONCs, AgNPs, and LSPR effect that efficiently promoted the absorption and generation of the photoinduced electrons and holes. It demonstrated that the existence of photocatalyst played an important role in improving the disintegration efficacy. Interestingly, in comparison with pristine TiONCs, TiONCs grafted with AgNPs exhibited a higher decomposition of organic dyes under both UV light and visible light illumination. As depicted in Figure 5(a,b), it was noteworthy to mention that among various composite photocatalysts, TiONCs@Ag-1.0 photocatalysts the highest MO decomposition efficiency of 85% and 62% under UV and visible light illumination, respectively. With increasing Ag concentration caused a decrease in the photocatalytic performance due to the shielding effect and preventing the interaction of light to the photocatalyst that could be assigned to the reduction in the photocatalytic performance of TiONCs@Ag-1.5 under both UV and visible lights. These results exhibited that the combination of Ag and TiONCs was accountable for enhancing the photocatalytic efficacy under UV and visible light irradiation. The kinetic curves for the decomposition of organic dye were determined through the linearized first-order decay model ln (C/C)=kt, where C and C were corresponding to the absorbance of MO at the beginning time and reacting for a certain time t, respectively, and k was pseudo-first rate kinetic constant 26. The achieved data exhibited that there was a linear correlation between ln(C/C) and the illumination time, indicating that the disintegration of MO dye followed the first-order rate law under UV light and visible light as depicted in Figure 5(c,d). The reaction rate constants for the degradation of MO were found to be 0.0068 min, 0.0090 min, 0.0139 min, 0.0109 min, and for pure TiONCs, TiONCs@Ag-0.5, TiONCs@Ag-1.0, and TiONCs@Ag-1.5, respectively, under UV light. The estimated reaction rate constants were 0.0006, 0.0060, 0.0093, and 0.0069 min, respectively, under the visible light. The reaction rate constant value of TiONCs@Ag photocatalyst showed outstanding degradation of organic dye compared with pristine TiONCs, which was governed by i) the increase in the surface area based on TiO cubic structure and enhancement of absorption ability under irradiated condition based on the plasmonic effect of AgNPs; iii) improvement of charge transport phenomenon and prevention of charge recombination due to the establishment of a Schottky barrier between the Ag and TiO, leading to a superior performance of Ag decorated TiO NCs.

The photocatalytic activity of TiO2NCs and TiO2NCs@Ag photocatalyst through decomposition of MO organic dyes. photodisintegration behaviors and kinetics under UV light (a, c) and visible light irradiation (b, d) of the as-prepared photocatalyst.
DISCUSSION
In recent years, the anatase TiO cubic shapes exhibited an outstanding performance compared with nanotubes, nanoparticles because of their larger specific surface 27, 28, 29. Therefore, being grafted with AgNPs was favorable for the transportation and adsorption of organic substrates, leading to excellent photocatalytic performance of TiONCs@Ag structure. Based on above results, to further understand the photocatalytic performance, a photocatalytic reaction decomposition mechanism was proposed (Figure 6). Upon exposure to UV light (Figure 6(a)), TiO was excited and generated the charge carriers. The photogenerated electrons jumped to the CB and transferred to Ag NPs. These electrons did reduction reactions to form˙O− radical anions. Meanwhile, the photoinduced holes left behind at the VB and directly oxidized the absorbed HO to generate •OH radicals. Regarding visible light irradiation (Figure 6(b)), TiO did not excite due to the wide forbidden energy gap. Only the Ag NPs could strongly absorb the visible light depending on the LSPR effect to generate hot electrons. These photo-excited electrons transferred to CB of TiO and participated in redox reactions to form reactive species reduced to form˙O−radical anions. These generated reactive species with highly oxidative capability participated in the oxidative deterioration of organic dye. Based on the above results, the presence of Ag NPs improved the photocatalytic performance of TiO NCs under both UV light and visible light irradiation. This experiment showcased that the coupling of TiO NCs and Ag could provide a new insight into the decomposition of pollutants.

Schematic illustration of the charge transfer process in the TiO2NCs@Ag photocatalyst (a) under UV light irradiation; (b) under visible light irradiation.
CONCLUSION
In summary, a remarkable photocatalytic performance of solid-phase heterojunction photocatalysts for degradation of MO dye on TiONCs@Ag as a model reaction system based on the hydrothermal procedure and photo-reduction AgNO in the presence of UV light. The TiONCs@Ag specimens exhibited an effective degradation process of MO dye in both UV light and visible light irradiation with respect to that of the bare TiO. These results were obtained in the presence of AgNPs and the unique properties of TiO nanocubes. This excellent behavior was designated to the efficient creating interaction at junctions between TiO and AgNPs, leading to enhanced photocatalytic and effective charge carrier generation. The as-prepared TiONCs@Ag heterojunction provided not only a possible pathway to improve the photocatalytic efficiency under visible-light but also opened new prospects for designing 3-dimension photocatalysts.
COMPETING INTERESTS
The authors declare that there is no conflict of interest regarding the publication of this article.
AUTHORS’ CONTRIBUTIONS
Ton Nu Quynh Trang has constructed the present idea, carried out, and written the manuscript with support from Vu Thi Hanh Thu.
Le Thi Ngoc Tu conducted the experiments
Tran Van Man has supported analytical techniques.
All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This research is funded by the University of Science, VNU-HCM under grant number T2020-07.

