Gold nanoparticles enhanced fluorescence for highly sensitive biosensors based on localized surface plasmon resonance applied in determination C-reactive protein
- Faculty of Materials Science and Technology, University of Science, HoChiMinhCity, Viet Nam
- Vietnam National University, HoChiMinh City, Viet Nam
- Faculty of Materials Science and Technology, University of Science, Ho Chi Minh City, Viet Nam
- Center for Innovative Materials and Architectures (INOMAR), HoChiMinh City, Viet Nam
- Laboratory of Advanced Materials, University of Science, HoChiMinh City, Viet Nam
Abstract
Introduction: C-reactive protein (CPR) is known as an inflammation marker related to numerous pathology. Optical biosensor based on the fluorescence dyed is widely used in diagnosis. There are still limitations on the fluorescence signal detection due to the photobleaching effect. The localized surface plasmon resonance (LSPR) performed by gold nanoparticles (Au NPs) is testified for the enhancement of photo-signal gathered from the dye molecules.
Methods: In this study, Au NPs were used for their significant optical properties and biocompatibility additionally. The seed-mediated synthesis method provided stable NPs with all the essential qualities. A series of modification steps were done on a glass substrate before the bio-bonding for fluorescence-based sensing by a transmission mode (T-mode) detection system which is introduced for the first time in Viet Nam.
Results: The synthetic Au NPs in nanosphere structure evinced the absorbance at a maximum wavelength is 521 nm. All the followed alterations showed the accomplishment in forming the in need linking proved through the basic analysis methods. Finally, CRP with the Alexa 488 dye was observed for average at 4.8 folds of enhancement factor compared between the Au NPs coating and non-coating substrate detected by the T-mode system. The low coefficient of variation at under 0.7% appeared for the repeatability and stability of this sensor.
Conclusion: The completely modern approach of the T-mode system combined with the LSPR applied in fluorescence sensors enhanced is developed successfully. Also, the future prospect of this designed sensing method is promising by changing the materials' structures and ingredients.
Keywords: LSPR, gold nanoparticles, fluorescence enhancement, C-reaction protein, optical biosensors
INTRODUCTION
Label-based biosensors are widely applied in life science and beyond. One of the most powerful utilizations of that rapid and high-performance method is in biomedical fields such as diagnosis of infectious diseases, cancer detection, DNA determination1, 2, 3, . To increase the sensitivity for lower the test limit of detection (LOD), the potential metal nanoparticles (NPs) were used since their outstanding behavior in optical, electrochemical, plasmonic, and radiative properties4, 5, 6. In this paper, gold nanoparticle (Au NPs) synthesized by Turkevich method7 was investigated for its localized surface plasmon resonance (LSPR) effects in other signal enhancement for a labeled based optical biosensor system applied in C-reactive protein (CRP) detection.
Besides other featured optical properties that were mentioned even from the Lycurgus Cup's invention back to the 4 century A.D, localized surface plasmon resonance (LSPR) is an attractive optical phenomenon most found in the noble metal nanostructure. The exceptional ability proved in the absorption of the special incoming light then converts the photon energy into the electrons' oscillation before scattering it with another collective-wavelength outcome light8. Therefore, the optical occurrence consisting of photobleaching does not occur as if LSPR happened9. Based on the difference in the nanoparticles size, shape, ingredient, and interspace, a large adjustment of LSPR was provided mostly in the maximum absorbance wavelength under the excitation light10. Gold was used in our study since its chemical stability forces a wide range of applications and is currently being attended in biosensors11.
CRP is known as an inflammation marker significant aggregation in the liver and recommended testing in postoperative infection monitor cases, pathological issues determine such as lymphoma, intestinal hemorrhage, or rheumatoid arthritis and access to their treatment respond12. Further CRP quantitative studies were applied various methods, including electrochemical immunoassay (ECL), photothermal system (PTB), vertical flow immunoassay (VFA), and so on gained some remarkable results13.
This work aims to demonstrate the extraordinary improvement of luminescence signal exploits brought by gold nanoparticles aid for a potential optical biosensor. The Alexa 488 fluorophore molecules stained on CRP are eager to strengthen and stable under the assistance of nano metallic coating.
MATERIALS AND METHOD
Gold(III) chloride trihydrate (HAuCl.3HO, 99%), sodium citrate tribasic dihydrate (NaCtr), 1,4-Dioxane (CHO, 99%), succinic anhydride (SA, 99%), (3-Aminopropyl) triethoxysilane (APTES, 99%), 11-Mercaptoundecanoic acid (11-MUA, 95%) were purchased from Sigma-Aldrich, USA. Ethanol (CHOH, 99.5%) and methanol (CHOH, 99.5%) were provided from Fisher, USA. Sodium hydroxide (NaOH) was acquired from Guangdong Guanghua Sci-Tech, China. Polydimethylsiloxane (PDMS) was procured from Dow, Korea. C-Reactive Protein/CRP Antibody Alexa Fluor® 488 (CRP@Alexa 488) was obtained from Novus Biologicals, USA.
Preparation of gold nanoparticles (Au NPs)
The synthesis of Au NPs was completed by the seed-mediated method with NaCtr roled as the reductant for its rapid and simple protocol14. A colloidal solution of gold nano seed was quickly assembled at high temperatures by the ingredient reaction. The next stage of growing seed was continuous right after the cooling down period while the light was avoided to prevent photoreduction. The appropriate volume of grown solution was synthesized from the remaining scattering in the ultrasonic bath and finally stored in the required condition.
Preparation of the modified substrates
The clean glass substrates underwent a pre-set process of the CUTE plasma cleaner from Femto Science, Korea, for the silanol forming before being treated by 3% APTES in ethanol to fabricate amine-rich surfaces. Right after, two separate antibody bonding methods were performed. Figure 1a illustrates a five-step no nano metal coating process with the direct antibody linking to the carboxyl from SA treatment. On the other hand, Figure 1b shows the advanced Au NPs coating process by simply immersion in the nanoparticle storing condition. 11-MUA was used for carboxyl modification after the nanometal middle layer and responded to the CRP@Alexa 488 coupling.

The schematic of CRP@Alexa488 bonding on the glass substrate with and without Au NPs coating. The general processes included silanized, and amination was done on glass substrates by O2 plasma and 3% APTES modification methods. Then, a) the non-metallic coating was performed through carboxylated by SA and antibodies linking by immersion. In the meantime, b) the Au NPs coating with surveyed incubation time was completed before carboxylated by 11-MUA and antibodies linking by the same immersion method.
Fabrication of PDMS microfluidic LSPR device
A PDMS round-shaped mask was placed on the glass substrate after fabrication from mixing the base and the curing agent then heating up to solid15. The mask played a role in fitting the light pathway from the optical detection system and limiting chemical loss in the modification.
Analytical Methods
The optical property of the nanoparticles was analyzed by the V-730 Ultraviolet-visible spectroscopy (UV-Vis) from JASCO, in the range from 400 nm to 800 nm for determination of the LSPR accordant maximum absorbance wavelength. The accomplishment of the surface modification was assessed by Fourier-transform infrared spectroscopy (FTIR) by the Vertex 70v in transmission mode between 4000 cm and 500 cm, powder X-ray diffraction (PXRD) by the V8 Advance from Bruker from 35 to 80 degree, the Hitachi 4800 field emission scanning electron microscopy (FESEM) at the scale of 300 nm and the Euromex OX.2253 PLPHF fluorescence microscopy under the blue light mode with 1 s of exposure time.
RESULTS
The Au seed and grown Au NPs performed in the wine red while the seed was darker for its high concentration. Besides, both colloidal solutions were surveyed for the maximum absorbance wavelength by UV-Vis spectroscopy. They showed up at 520 nm for the seed and 521 nm for the 40 min synthesis nanoparticles in Figure 2.

The UV-Vis spectra of Au seed and 40 min synthesis Au NPs. The Au seed and Au NPs were kept in light-free and 4oC conditions before diluted to analyze and performed the absorbance at 520 and 521 nm, respectively.
A transmission mode of FTIR was used with each modified step’s outcome, including O plasma, APTES, SA, and 11-MUA treatment. The range of wavelengths from 4000 cm to 500 cm was observed to record the feature spectrum peaks. Figure 3 shows the FTIR spectra from the measurement of the bonding interaction's oscillation on the silica surface.

The FTIR spectra of the modified substrates. The same modification processes were repeated on silica monocrystalline silicon substrates for the transmission analysis mode of the bonding interaction after a) O2 plasma treatment, b) APTES treatment, c) SA treatment and, d) 11-MUA treatment. The results represented the corresponding stretching and bending vibration of the required organic group, such as SiO-H of silanol, N-H of amine, and -COOH of carboxylic.
The crystallite structure and particle shape of the Au NPs coating surface were examined by the XRD analysis in the angle range from 35 to 80 and the FESEM at the scale of 300 nm. In Figure 4a, four peaks presented to four orientations were recorded at about 38, 43, 62, and 78 which was fully described in the Joint Committee's database on Powder Diffraction Standards, USA (JCPDS no. 00-004-0784)16.
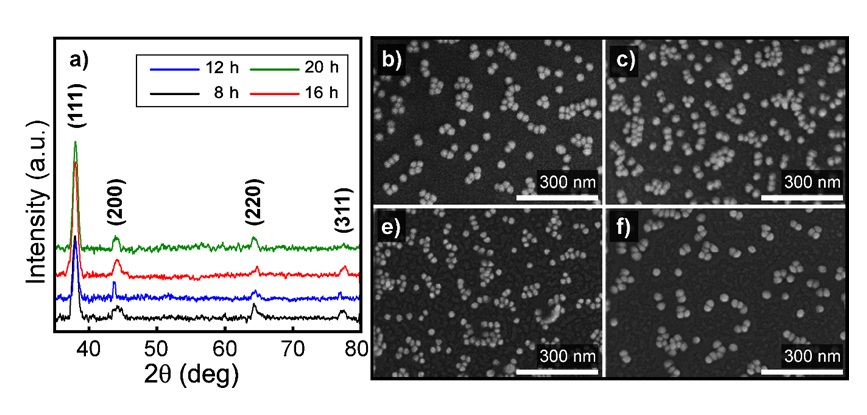
The XRD spectra and FESEM capture of Au NPs coating glass by time. A catalyze of Ni-filtered Cu in the X-Ray diffraction, and a 300 nm length scale of magnification in the FESEM was used for: a) investigating the crystallite structure and particles appearance after the Au NPs immersion of b) 8, c) 12, d) 16 and, e) 20 h. The XRD result of 4 particular orientations of gold crystallite at around 38o, 43o, 62o, and 78o, then clearly spherical nanostructure of them at the size about 20 to 40 nm under FESEM analysis was proved the successful synthesis and coating.
The Alexa Fluor 488 stained CRP anti-mouse IgG2a incubated over 5 h on two modified types of glass substrate were observed under a white light mode considered as the blank in Figure 5a-5c, and 1 s exposed blue light mode as the verification of coupling bio-factor appearance in Figure 5b-5d. In one hand of Figure 5a-b, the model bonding procedure by SA was performed with fewer and fewer radiation markers localized by the green dots and streaks in which also told an uneven distribution of molecules. On the contrary, a moister, brighter, and denser behavior showed up on the Au NPs inclusion surface via the comparison captures in Figure 5c-d without changing the biological incubation and fluorescence analysis.
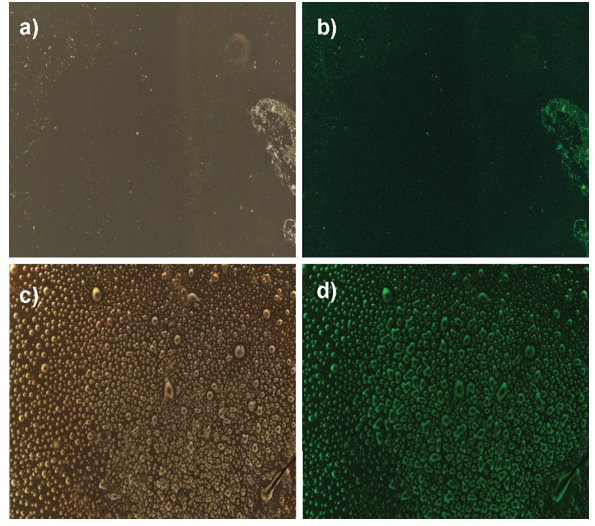
The fluorescence microscopy images of CRP@488 bonding samples. The different light modes were used in recognition of the fluorescence dyed antibodies on no Au NPs coating glass under a) white light, b) 1 s exposure blue light, and Au NPs coating glass under c) white light, and d) 1 s exposure blue light. Under the excitation blue light, the dye of antibodies succeed linking on the modified Au NPs coating substrates was performed its enhanced fluorescence compared to the non-coating.
An optical system based on the T-mode (transmission mode) described in the previous study17 was used for fluorescence detection and transform into an electric signal. The enhancement factor (EF) of the Au NPs coated the (1) equation calculated substrates in the mentioned reference. Figure 6 illustrates the fluorescence intensity and enhancement factor recorded from the CRP@Alexa 488 bonding on the comparison coating and non-coating substrate with six different concentrations listed in

The fluorescence intensity and enhancement factor of CRP@488 detection by Au NPs coating substrates. The detecting solution of antibodies was prepared into different concentrations of 0.01, 0.03, 0.05, 0.1, 0.3 and 0.5 mg/mL. a) The fluorophore signal gathered from the compared substrates of Au NPs coating and non-coating was recorded under excitation light. b) The calculated enhanced factor at about 4.8 folds throughout the detection of 6 examined CRP@488 solutions.
The EF and CV of the examined samples were based on the antibody concentration.
|
CRP concentration (mg/mL) |
Fluorescence intensity of non-Au NPs coating (µW) |
Fluorescence intensity of Au NPs coating (µW) |
Enhancement factor (fold) |
Coefficient of variation (%) |
|
0.01 |
0.20024 |
0.94225 |
4.9 |
0.06 |
|
0.03 |
0.23321 |
1.06793 |
4.7 |
0.03 |
|
0.05 |
0.27692 |
1.232415 |
4.6 |
0.7 |
|
0.1 |
0.29209 |
1.372065 |
4.8 |
0.3 |
|
0.3 |
0.30839 |
1.464335 |
4.8 |
0.04 |
|
0.5 |
0.33479 |
1.575505 |
4.8 |
0.3 |
DISCUSSION
Due to the Mie theory18 about the effects of nanoparticle size on its optical property, the recorded surface plasmon absorbance spectrum at about 520 nm in the UV-Vis range was coincidental to other studies about the LSPR application in biosensor19. Furthermore, the described color was presented to the nanoparticles' size in the range between 20 - 40 nm, which was pointed out from other researches20 and fit with the UV-Vis result.
Four repaired samples had the Si-O-Si asymmetric vibration at around 1060 cm in common since the silica substrate21. The broadband at the range of 3485 cm of Figure 3a is related to the silanol groups' stretching isolated vibration for being treated by O plasma22. In the case of APTES treatment, the primary amine groups' stretching mode may be noticed at the band of 3362 cm. Therefore, its bending and wagging mode also from the N-H bonding was shown up at 1562 and 759 cm respectively, in Figure 3b23. The SA and 11-MUA method to form the carboxylate group onto the furthest side of the surface were illustrated in Figure 3c-d. The spectrum of both revealed the broadband of hydroxyl groups stretching mode at around 3410 cm and 3458 cm. The other stretching mode vibrations of C=O and C-O oscillations illustrated at the peaks of 1695, 1701, and 1450, 1467 cm24. The double peaks at 2922 and 2850 cm noticed in Figure 3d were assigned to the alkynes bonding from the MUA substances structure, which were the asymmetric and symmetric stretching modes. The familiar peaks are either APTES and SA spectrum but single and weaker since the shorter carbon chains25, 26.
Otherwise, the nanospheres showed up on the coated after being affected by the microscopy electron beam. In detail, the face-centered cubic (FCC) structure of pure crystalline gold has appeared for the Bragg reflection. The (111) orientation got the strongest intensity of all, while the other recorded three, including (200), (220), and (311) had an approximately equal intensity. However, the most promising signal over the different immersion times was in the 16 h Au NPs coating surface, although the spectrum underwent a trend of increase. By using the Scherrer equation in calculating the crystalline size27 after the spectra of the (111) orientation, the size is more or less 21.5 nm. It also coincided with the FESEM captures considered by the nanoparticle's uniformity and the surface arrangement. By comparison with the nanoscale, the sphere's size is between 20 nm and 40 nm and consistent with the other analysis as UV-Vis and XRD. Figure 4b of 8 h immersion of Au NPs shows a lower density of nanospheres formed on the surface than the others. Figure 4c-e speak for 12, 16, and 20 h immersion, the selected 16 h meet the need for a convenient experimental time and the required about the space distributed.
As the desired LSPR effect on the optical signal enhancement from the advanced metal nanoparticles, the result assumed from the visible images presents a successful immobilization through both fabrications within the major domination in magnification light emission of the synthesized gold nanoparticles. Figure 5b-d shows the photo taken to confirm the fluorescence's green color from the fluorophores Alexa 488 conjugated with the CPR used in two different configuration coating (pristine glass and AuNPs-coated glass). This allows the visual check-up of the fluorescence under ultraviolet light excitation and leads us to determine the CPR concentration appropriate for clarifying the significant difference between the no coat and AuNPs-coat amplifications.
An similar trend of the increase happened in both methods, but a sharper shape can be mentioned by the Au NPs coating one. The no Au coating samples give the power under 0.4 µW while the coated reaches the peaks from 1 to 1.5 µW. Figure 6b presents the enhancement factor caused by the LSPR effect as desired, and the average 4.8 fold multiplication has occurred. The maximum enhancement resulted is in 0.01 mg/mL binding antibody and dropped slightly when it comes to 0.05 which the experimental manipulation may cause. It almost remained throughout the 10 times higher and more concentration that may be responsible for the limited carboxyl modified groups in the chamber work area. The LOD for this detection experiment can be accounted for 0.01 mg/mL, which is 100 times lower than the standard of CRP low-risk announcement at 1 mg/L (published in 2003 by The American Heart Association and U.S. Centers for Disease Control and Prevention), and much higher than the earlier research based on lossy-mode resonance (LMR) fiber devices28. The coefficient of variation (CV) was shown in
CONCLUSIONS
The gold nanoparticles synthesized from the seed-mediate method was proved the strong LSPR effect on the enhancement of the fluorescence signal. Besides, a simple and effective modification protocol was performed aimed to coat Au NPs and to bond the biofactor. The accomplished substrate pattern was built prepared for sensing through the curtain analysis of FTIR, XRD, FESEM, and fluorescence microscopy. As expected, a high sensitivity optical biosensor based on LSPR was designed and fabricated that got the remarkable EF at 4.8 folds with a low CV at 0.7%. There is an auspicious prospect in the ability of other noble metals utilization, switching into others structure or size, and a combination of various optical phenomena for the applications.
ABBREVIATIONS
11-MUA: 11-Mercaptoundecanoic acid
APTES: 3-(Triethoxysilyl)Propylamine
Au NPs: Gold nanoparticles
CRP: C-Reactive protein
CV: Coefficient of variation
DI: Deionized water
FESEM: Field emission scanning electron microscope
FTIR: Fourier-transform infrared spectroscopy
LSPR: Localized surface plasmon resonance
PDMS: Polydimethylsiloxane
SA: Succinic anhydride
UV-Vis: Ultraviolet-visible
WCA: Water contact angle
XRD: X-Ray diffraction
COMPETING INTEREST
The author(s) declare that they have no competing interests.
AUTHORS’ CONTRIBUTION
All the authors read and corrected the submitted final version.
Phuong Que Tran Do and Tran Duc Trung has conceived experiments design, analyzed data, carried out, and written the manuscript with support from Dr. Nhu Hoa Thi Tran.
Bach Thang Phan, Hanh Kieu Thi Ta, Ngoc Xuan Dat Mai carried out the experiments in group
Lai Thi Hoa, Thanh Van Thi Tran, Dung Van Hoang have supported the analysis techniques. Dr. Nhu Hoa Thi Tran revised and corrected the manuscript.
ACKNOWLEDGEMENT
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.03-2019.379. I would like to gratefully acknowledge the Vietnam National University in Ho Chi Minh City to Center for Innovative Materials and Architectures (Laboratory for Optics and Sensing).

