Phenolic compounds from the lichen Parmotrema tinctorum
- Department of Science, Dong Nai University, Dong Nai Province
- Department of Organic Chemistry, University of Science, National University – Ho Chi Minh City, Ho Chi Minh City
- Faculty of Environmental Science, Sai Gon University, Ho Chi Minh City
Abstract
Introduction: The metabolites of lichens concentrated depsidones, depsides, and diphenyl ethers were possessed antibiotic, antifungal, antiviral, antitumor, and anticancer activities. Parmotrema tinctorum (Despr. ex Nyl.) Hale, a species of foliose lichen, is widely distributed in Lam Dong province, Vietnam. Herein, this paper describes the isolation and structure elucidation of seven compounds isolated from this lichen. Methods: Phytochemical investigations of the ethyl acetate extract of the lichen P. tinctorum led to the isolation of seven pure compounds. Their chemical structures were elucidated by extensive HR-ESI-MS and NMR spectroscopic analysis and comparison with previously published data. Results: Seven compounds, namely orcinol (1), orsellinic acid (2), methyl orsellinate (3), methyl heamatomate (4), lecanorin (5), lecanoric acid (6), and gyrophoric acid (7). These compounds were determined the α-glucosidase inhibitory activity. Conclusions: Compound 7 was determined for the first time in P. tinctorum, and this was also the first time these compounds were determined the α-glucosidase inhibitory activity.
INTRODUCTION
The development of an α-glucosidase inhibitor derived from natural products is an important contribution to the treatment of diabetes.1, 2 was used as an edible spice for flavoring food in Kerela, India.3 The methanolic extract of exhibited the anti-arthritic potential on experimental rats.4 For the purpose of searching for new classes of -glucosidase inhibitors, we reported the isolation and structural elucidation of seven compounds from the ethyl acetate extract of the lichen (Nyl.) Hale and the -glucosidase inhibitory activity of these compounds.
MATERIALS AND METHODS
General experimental procedures
The HR–ESI–MS was recorded on a HR–ESI–MS MicrOTOF–Q mass spectrometer. The H-NMR 500 (MHz) and C-NMR (125 MHz) spectra were recorded on a Bruker Avance 500 spectrometer. Thin-layer chromatography (TLC) was carried out on precoated silica gel 60 F254 or silica gel 60 RP–18 F254S (Merck), and the isolated compounds were visualized by spraying with 10% HSO solution followed by heating. Gravity column chromatography was performed on silica gel 60 (0.040–0.063 mm, Himedia).
Plant material
The thalli of the lichen (Nyl.) Hale was collected at Lam Dong province, Vietnam, in April 2020 and authenticated by Dr. Vo Thi Phi Giao, Faculty of Biology, University of Science, National University – Ho Chi Minh city. A voucher specimen (No US–B025) was deposited in the Faculty of Chemistry, University of Science, National University - Ho Chi Minh City.
Extraction and isolation
The powder of the lichen (3.15 kg) was exhaustively extracted with acetone at room temperature. After filtrated, the acetone solution was evaporated at the reduced pressure to provide the crude acetone extract (350.0 g), which was subjected to silica gel solid phase extraction and eluted consecutively with the solvents with various polar to afford -hexane extract (H, 19.05 g), chloroform extract (C, 119.72 g), ethyl acetate extract (EA, 164.58 g) and methanol extract (M, 31.08 g).
The extract EA (164.58 g) was applied to silica gel column chromatography and eluted with the solvent systems of -hexane-ethyl acetate (stepwise, 7:3, 5:5, 0:10) then methanol to give 9 fractions, coded M1-M10. Fraction M1 (606.8 mg) was divided into two sub-fractions M1.1 (284.6 mg) and M1.2 (284.6 mg), by silica gel column chromatography, using the mobile phase as -hexane-chloroform (7:3, v/v). Sub-fraction M1.1 was rechromatographed by silica gel column chromatography, eluted with -hexane-chloroform (7:3, v/v) to yield 1 (12.0 mg). The same procedure for subfraction M1.2 (487 mg) was conducted, eluting with -hexane-chloroform (5:5, v/v) to obtain 2 (14.3 mg), 3 (18.7 mg) and 4 (10.3 mg). Fraction M2 (16.2 g) was subjected to silica gel column chromatography and eluted by chloroform-methanol (98:2, 95:5, 9:1) to give 5 (9.5 mg), 6 (17.4 mg) and 7 (11.2 mg).
-Glucosidase inhibition assay
The -glucosidase inhibitory activity was evaluated on all compounds according to the method of Kim5. A reaction mixture containing 3 mM -nitrophenyl--D-glucopyranoside (25 μL), 0.2 U/mL -glucosidase (25 μL) in 0.01 M phosphate buffer (pH = 7.0) and the sample solution (625 μL) was incubated at 37 °C for 30 min and stopped by adding 0.1 M NaCO (375 μL). Absorbances were recorded at 401 nm. One unit of -glucosidase activity was defined as the amount of enzyme liberating -nitrophenol (1.0 μM) per min. Acarbose was used as the positive control.
RESULTS
The chemical investigation on the extract EA of the lichen P.tinctorum led to the isolation of seven compounds by the use of efficient separation techniques, including orcinol (1), orsellinic acid (2), methyl orsellinate (3), methyl heamatomate (4), lecanorin (5), lecanoric acid (6), and gyrophoric acid (7). Their C-NMR data were performed in
-
Orcinol (1): Colorless needles, mp 107 oC. HR-ESI-MS (positive mode)
m/z 125.0602 [M+H]+ (calcd. for C7H8O2+H 125.0603). The 1H-NMR data (CDCl3,δ ppm,J in Hertz): 6.23 (2H,d , 1.5, H-1 and H-5), 6.17 (1H,t , 2.0, H-3), 5.09 (2H,s , -OH) and 2.24 (3H,s , 6-CH3). The 13C-NMR (CDCl3) was presented inTable 1 . -
Orsellinic acid (2): Colorless needles, mp 184 oC. HR-ESI-MS (negative mode)
m/z 167.0346 [M–H]–(calcd. for C8H8O4-H 167.0345). The 1H-NMR data (DMSO-d6 ,δ ppm,J in Hertz): 6.04 (1H,d , 2.0, H-5), 6.02 (1H,d , 2.0, H-3) and 2.41 (3H,s , 6-CH3). The 13C-NMR (DMSO-d6 ) was presented inTable 1 . -
Methyl orsellinate (3): Colorless needles, mp. 143-144 oC. HR-ESI-MS (positive mode)
m/z 183.0668 [M+H]+ (calcd. for C9H10O4+H 183.0658). The 1H-NMR data (CDCl3,δ ppm,J in Hertz): 11.77 (1H,s , 2-OH), 6.28 (1H,d , 2.5, H-3), 6.23 (1H,d , 2.5, H-5), 3.92 (3H,s , -OCH3) and 2.48 (3H,s , 6-CH3). The 13C-NMR (CDCl3) was presented inTable 1 . -
Methyl heamatomate (4): Colorless needles, mp 146 oC. HR-ESI-MS (negative mode)
m/z 209.0449 [M-H]- (calcd. for C10H10O5-H 209.0450). The 1H-NMR data (Acetone-d6 ,δ ppm,J in Hertz): 12.84 (1H,s , 2-OH), 12.24 (1H,s , 4-OH), 10.24 (1H,, -CHO), 6.30 (1H, , H-5), 3.93 (3H, , -OCH3), 2.48 (3H, s , -CH3). The 13C-NMR (Acetone-d6 ) was presented inTable 1 . -
Lecanorin (5): White amorphous powder. HR-ESI-MS (negative mode)
m/z 273.0773 [M-H]- (calcd. for C15H14O5-H 273.0763). The 1H-NMR data (CDCl3,δ ppm,J in Hertz): 11.42 (1H,s , 2-OH), 6.59 (1H,s , H-5'), 6.58 (1H,s , H-1'), 6.50 (1H,s , H-3'), 6.32 (1H,s , H-3), 6.31 (1H,s , H-5), 2.62 (3H,s , 6-CH3) and 2.33 (3H,s , 6'-CH3). The 13C-NMR (CDCl3) was presented inTable 1 . -
Lecanoric acid (6): Colorless needles, mp. 184 oC. HR-ESI-MS (negative mode)
m/z 317.0663 [M-H]- (calcd. for C16H14O7-H 317.0662). The 1H-NMR data (Acetone-d6 ,δ ppm,J in Hertz): 11.13 (1H,s , 2-OH), 6.77 (1H,s , H-3), 6.74 (1H,s , H-5), 6.39 (1H,s , H-5'), 6.30 (1H,s , H-3'), 2.63 (3H,s , 6-CH3) and 2.59 (3H,s , 6'-CH3). The 13C-NMR (Acetone-d6 ) was presented inTable 1 . -
Gyrophoric acid (7): Colorless needles, mp. 225 oC.. HR-ESI-MS (negative mode)
m/z 467.0989 [M-H]- (calcd. for C24H20O10-H 467.0978). The 1H-NMR data (Acetone-d6 ,δ ppm,J in Hertz): 11.13 (1H,s , 2-OH), 6.87 (1H,s , H-3'), 6.87 (1H,s , H-3'), 6.80 (1H,s , H-3), 6.76 (1H,s , H-5), 6.85 (1H,s , H-5'), 6.39 (1H,s , H-5''), 6.31 (1H,s , H-3''), 2.66 (3H,s , 6'-CH3), 2.65 (3H,s , 6-CH3) and 2.61 (3H,s , 6''-CH3). The 13C-NMR (Acetone-d6 ) was presented inTable 1 .
DISCUSSION
Compound 1 was isolated as colorless needles; its molecular formula was determined as CHO through its pseudo molecular ion peak at 125.0602 [M+H] in the HR-ESI-MS spectrum. The H-NMR spectrum data of compound 1 gave signals of one methyl group at 2.24 (3H, ), two hydroxyl protons at 5.09 (2H, , -OH) and three aromatic methine protons at 6.23 (2H, , 1.5) and 6.17 (1H, , 2.0).The C-NMR spectrum data showed the resonances of five signals, including one methyl group at 21.5 (C-7) and four aromatic methine carbons at 100.1, 108.9 , 141.1 and 156.8 (
Compound 2 was obtained as colorless needles. The spectral features of 2 were closely similar to those of 1, except for the absence of one aromatic proton and the presence of carboxyl group in 2. The position of the carboxyl group was determined via HMBC correlations from the methyl protons at 2.41 (H-8) to carbon signals C-1 ( 109.4), C-5 ( 100.3), and C-6 ( 142.5) and from the aromatic proton at 6.02 (H-3) to carbon signals C-1 ( 109.4), C-2 ( 160.4) and C-4 ( 165.3) (Figure 1). The NMR data of 2 showed good compatibility with those of orselinic acid in the literature6, 7, so the structure of compound 2 was suggested as orselinic acid.
The comparison NMR data of 2 and 3 showed that the latter possessed one more methoxy group. This was evidenced by the presence of a signal of methoxy protons adjacent to a carboxyl group at 3.92 (H-9). Furthermore, the molecular weight of compound 3 is 14 mass units larger than that of compound 2, which could be attributed to a methoxy group in 3. Base on the above NMR data analysis as well as the HR-ESI-MS spectrum of 3, the chemical structure of 3 was determined as methyl orselinate.8
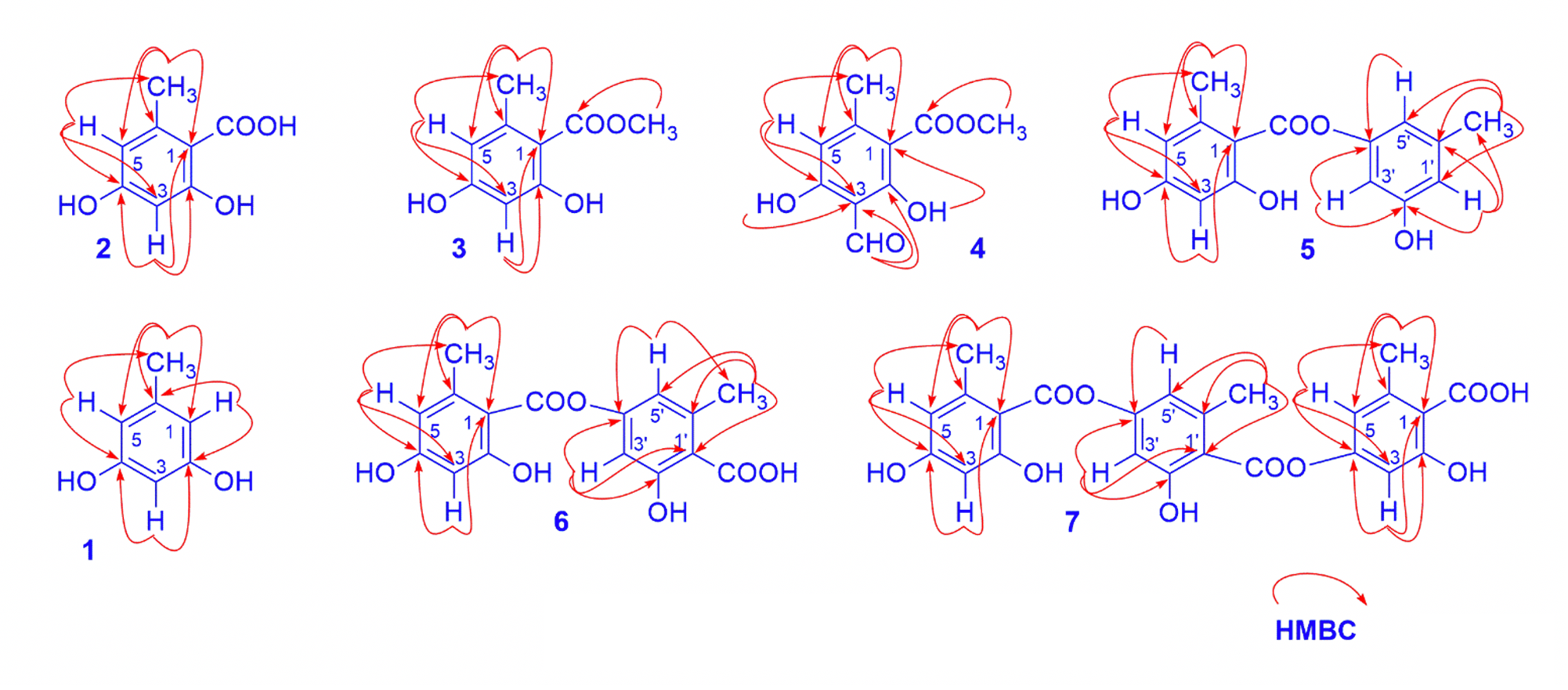
Key HMBC of isolated compounds
The comparison of chemical shift values of 4 with corresponding ones of 3 also showed that they had the same structure. The difference was that an aromatic proton at C-3 in 3 was replaced by a formyl group in 4. This was evident by the absence of an aromatic proton and the observation of a formyl proton signal and a carbonyl carbon signal in the low field zone at 10.24/194.8. The position of this formyl group was clarified on the basis of the HMBC correlation between the formyl proton (H-8) to C-2 ( 168.5) and C-3 ( 109.1). It corresponded to the molecular formula CHO, which was determined through its pseudo molecular ion peak at 209.0449 [M-H]. By comparing these data with those published in the literature,84 was identified as methyl heamatomate.
Compound 5 was a depside. Its molecular formula was determined as CHOthrough its pseudo molecular ion peak at 273.0773 [M-H] (calcd. for CHO-H 273.0763). The H-NMR spectrum data of compound 5 displayed signals of a chelated hydroxyl group at 11.42 (1H, ), five aromatic protons at 6.59, 6.58, 6.50, 6.32 and 6.31 (1H each, ), and two methoxyl groups at 2.62 and 2.33 (3H each, ). The C-NMR exhibited 15 carbon signals, consisting of two methyl carbon signals ( 21.6 and 24.7), twelve aromatic carbons ( 101-167 ppm), and one carboxyl carbon signals ( 170.5) (
Compound 6 was also a depside with similar NMR signals as those of 5, except for the displaying of carboxyl group at C-1 instead of an aromatic proton in 5. The mass value of compound 6 has 44 more atomic mass units than those of 5, which showed the presence of a carboxyl group. Base on the good compatibility of its HR-ESI–MS and NMR data with those reported in the literature7, 9, 6 was proposed to be lecanoric acid.
Compound 7 was a depside. The NMR data of compound 7 displayed signals of three orselinic units with six aromatic protons, three methyl groups in H-NMR spectrum and 24 carbon signals in C-NMR spectrum (Table1). Furthermore, the HR-ESI–MS spectrum of compound 7 showed a pseudomolecular ion peak at 467.0989 [M-H] calcd. for CHO-H 467.0978, therefore 7 was indicated as gyrophoric acid.9
The inhibitory effect against α-glucosidase of some isolated compounds of was tested. The results of tested compounds 4 displayed high-potency inhibitors with IC50 values in 38.9 μM, compared with the control acarbose IC50 214.5 μM. The pioneered results of evaluating the inhibitory effect against α-glucosidase were also presented in
|
No |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
1 |
108.9 |
109.4 |
105.7 |
105.4 |
105.0 |
111.6 |
112.4 |
|
2 |
156.8 |
160.4 |
165.4 |
168.5 |
166.2 |
165.4 |
165.5 |
|
3 |
100.1 |
100.3 |
101.4 |
109.1 |
101.6 |
109.4 |
109.2 |
|
4 |
156.8 |
165.3 |
160.7 |
167.1 |
161.4 |
155.0 |
154.8 |
|
5 |
108.9 |
100.3 |
111.6 |
112.4 |
112.0 |
117.2 |
116.9 |
|
6 |
141.1 |
142.5 |
144.1 |
153.4 |
150.0 |
144.8 |
144.8 |
|
7 |
21.5 |
173.0 |
172.3 |
172.6 |
170.5 |
170.4 |
169.0 |
|
8 |
23.4 |
24.4 |
194.8 |
24.7 |
23.9 |
23.9 | |
|
9 |
52.0 |
24.9 | |||||
|
10 |
52.9 | ||||||
|
1' |
114.8 |
104.5 |
113.5 | ||||
|
2' |
150.8 |
166.8 |
163.0 | ||||
|
3' |
106.0 |
101.9 |
109.5 | ||||
|
4' |
156.6 |
164.3 |
155.0 | ||||
|
5' |
114.4 |
112.9 |
117.5 | ||||
|
6' |
141.1 |
144.6 |
143.2 | ||||
|
7' |
21.6 |
174.0 |
169.0 | ||||
|
8' |
24.4 |
22.9 | |||||
|
1'' |
104.8 | ||||||
|
2'' |
166.8 | ||||||
|
3'' |
101.9 | ||||||
|
4'' |
164.3 | ||||||
|
5'' |
113.0 | ||||||
|
6'' |
144.8 | ||||||
|
7'' |
170.4 | ||||||
|
8'' |
24.4 |
|
No |
Compound |
IC50a (μM) ± SD |
|
1 |
Orcinol |
>250 |
|
2 |
Orselinic acid |
>250 |
|
3 |
Methyl orselinate |
>250 |
|
4 |
Methyl heamatomate |
38,9 |
|
5 |
Lecanorin |
>250 |
|
6 |
Lecanoric acid |
>250 |
|
7 |
Acarbose |
214.5 |
CONCLUSION
From the extract EA of the lichen collected in Di Linh district, Lam Dong province, using various chromatographic methods, seven phenolic compounds were isolated. Their structures were determined as orcinol (1), orsellinic acid (2), methyl orsellinate (3), methyl heamatomate (4), lecanorin (5), lecanoric acid (6), and gyrophoric acid (7). Among them, compounds 7 were reported for the first time in such species. This was the first time these compounds were determined the α-glucosidase inhibitory activity. This is remarkable for our further research.
ABBREVIATIONS
HR-ESI-MS: High resolution- Electrospray ionization-Mass spectrometry
H NMR: Proton nuclear magnetic resonance
C NMR: Carbon-13 nuclear magnetic resonance
HMBC: Heteronuclear multiple bond correlation
: singlet
: doublet
COMPETING INTEREST
The authors declare no competing financial interest.
AUTHORS’ CONTRIBUTION
Huynh B.L.C has contributed in conducting experiments, acquisition of data, interpretation of data. Bui V.M, Phan T.Q.N interpreted NMR and MS data as well as searched the bibliography Nguyen K. P. P. gave final approval of the manuscript to be submitted.
Corresponding author: Dr. Huynh Bui Linh Chi, Dong Nai University, 03 Le Quy Don, Tan Hiep district, Bien Hoa city, Dong Nai province. Email : hainhanchi@yahoo.com.vn.
ACKNOWLEDGMENT
We would like to thank Dr. Vo Thi Phi Giao for lichen identification.

