Improvement of antibacterial efficacy using antibiotic encapsulated silica-containing redox nanoparticles
- Faculty of Biology and Biotechnology, University of Science Ho Chi Minh city
- Vietnam National University Ho Chi Minh city
- School of Biomedical Engineering, International University Ho Chi Minh city
- 1. Faculty of Biology and Biotechnology, University of Science Ho Chi Minh city
- 2. Vietnam National University Ho Chi Minh city
- School of Biotechnology, Hong Bang International University
Abstract
Purpose: Improving solubility and antibacterial efficiency of cephalothin by using silica-containing redox nanoparticle (siRNP) as a system to encapsulate and deliver this hydrophobic antibiotic.
Methods: siRNP was synthesized by assembling amphiphilic block copolymers possessing a reactive oxygen species scavenging nitroxide radical and drug absorptive silica moieties in a hydrophobic side chain. Cephalothin, a hydrophobic antibiotic, was encapsulated into siRNP (cephalothin@siRNP) by mixing and dialysis methods. Antibacterial activity of cephalothin@siRNP against Staphylococcus aureus (S. aureus) và Escherichia coli (E. coli) was evaluated by the agar diffusion method.
Results: The average size of siRNP and cephalothin@siRNP was 43.83 nm and 50.15 nm, respectively. After encapsulation in siRNP, the solubility of cephalothin was improved compared to cephalothin in an aqueous solution. The result showed that in vitro antibacterial activities of cephalothin and cephalothin@siRNP had no statistical difference after 24 h incubation on agar plates on both S. aureus and E. coli. However, after an extended incubation time, regrowth of E. coli colonies in the inhibitory zone was found in cephalothin treated plate. Interestingly, E. coli regrowth was significantly reduced in plates treated with cephalothin@siRNP.
Conclusion: In this study, siRNP successfully encapsulated cephalothin and enhanced the solubility of this drug. The antibacterial activity of cephalothin is prolonged when encapsulated in siRNP, which suppressed the reccurrence of E. coli colonies. Cephalothin@siRNP has the potential to inhibit antibiotic resistance.
INTRODUCTION
Nowadays, indiscriminate use of antibiotics has increased the uncontrollable development of many multidrug resistance (MDR) bacteria, becoming one of the most dangerous threats to public health. Infection of MDR into the community lead to increased morbidity, mortality, higher expenditures for health care, and grade of using antibiotics1. Although many types of antibiotics have been developed, demand for antibacterial infection treatment are still an urgent need as the number of resistance of bacterial strains is increasing2. One of the main bacterial resistance mechanisms has been reported to be associated with the overproduction of reactive oxygen species (ROS)3. Under microbial infection , not only inflammatory response but also the use of antibiotics induce ROS overproduction. ROS overproduction might induce gene mutation, which activated the bacterial antibiotic resistance mechanism4. On the other hand, several antibiotics such as cephalothin, erythromycin, rifamycins, gentamycin, kanamycin… present a low bioavailability due to low water solubility, low stability under physiological environments5. Therefore developing new efficacy antibacterial method to improve bioavailability is urgently necessary. In recent years, there has been a growing trend of enhancing the efficacy of available antibiotics by using nanomaterials to carry and deliver them to improve their activities and solubility6. However, conventional nanoparticles have exhibited a low drug loading capacity without or with low ROS scavenging activity. Previously, we developed silica-contaning redox nanoparticle (siRNP) with ROS scavening from nitroxide radical and drug absorption silica moieties in the core of the nanoparticle7. siRNP with core-shell form micellar nanoparticle in aqueous media was synthesized from an amphiphilic copolymer composed of hydrophilic polyethylene glycol (PEG) and hydrophobic poly(silanoaminomethylstyrene--poly(4-(2,2,6,6-tetramethylpiperidine-1-oxyl) aminomethylstyrene) (siPMNT) (Figure 1). A stable nitroxide radical is covalently bonded with a hydrophobic chain via an amine linkage to scavenge ROS. At the same time, silica moieties form cross-linking to stabilize the structure of the nanoparticle and increase its loading drug capacity7. As an antibiotic model in this study, cephalothin, a generation of semi-synthetic antibiotic belonging to the cephalosporin group, kills and inhibits the bacterial growth by interrupting the synthesis of bacterial cells wall. However, low bioavailability of poorly water solubility and antibiotic resistance are the main challenges for using this antibiotic 8. This study aims to evaluate the improvement of cephalothin solubility by loading cephalothin into siRNP (cephalothin@siRNP) and investigating the antibacterial activity.
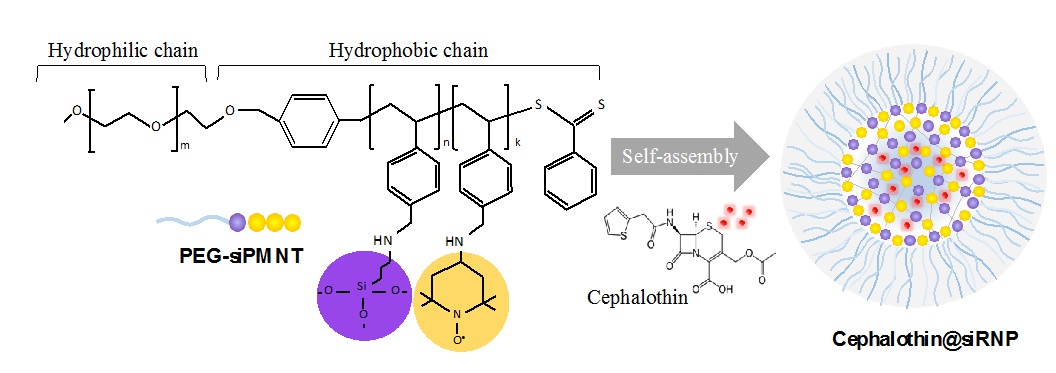
Structure of silica-contaning redox nanoparticle (siRNP) and cephalothin-loading siRNP (cephalothin@siRNP). siRNP was prepred by self-assembly of an amphiphilic block copolymer (PEG-siPMNT), which contains the ROS scavenging nitroxide radical moieties and drug absoption silica moieties. Cephalothin was encapsulated in the core of siRNP via the hydrophobic interaction and absorption on the silica.
MATERIALS AND METHODS
Synthesis of siRNP and cephalothin@siRNP
The silica-containing redox nanoparticle (siRNP) was prepared from amphiphilic PEG-siPMNT by dialysis, as described previously7. Briefly, 0.5 mL of PEG-siPMNT (60 mg/mL) was added to 0.5 mL of dimethylformamide (DMF, Wako Chemicals, Japan) and stirred well. The mixture was then transferred to a dialysis membrane (Spectra/Por, molecular-weight cutoff size 3500; Spectrum Laboratories, CA) and dialyzed against water for 24 h. Similarly, cephalothin@siRNP was prepared by dissolving cephalothin and PEG-siPMNT at a ratio of 1:10 in DMF and dialysis with the same protocol9.
Mesurement of nanoparticle size
Dynamic light scattering (DLS) measurement was used to evaluate particle sizes of siRNP and cephalothin@siRNP using Zetasizer Nano ZS (Malvern Instruments, Ltd., Malvern, UK) equipped with a 4-mW He-Ne ion laser ( = 633 nm). The measurements were conducted at 25 °C at a detection angle of 173°. siRNP was also observed by transmission electron microscope (TEM) imaging using a JEOL JEM-1400 instrument operated at 80 kV. The imaging samples were prepared by mounting a drop-cast onto a carbon-coated Cu grid and allowing it to dry in the air 7.
Evaluation of the DPPH free radical scavenging capacity of siRNP
The 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH, Tokyo Chemical Industry, Japan) free radical assay was conducted based on an electron-transfer that produces a violet solution in methanol. This free radical is reduced in the presence of an antioxidant molecule and leads to discoloration of the DPPH solution10. In this experiment, siRNP sample was diluted in water according to different concentrations with Tempo, and water served as the positive and negative control, respectively. A volume of 3 mL DPPH 0.1 mM in methanol was added to 2 mL sample followed by incubation in the dark for 30 mins. Absorbances were measured at 517 nm by Spectro–UV11 UV–Vis spectrometers (MRC Laboratory Instruments, Ltd, Israel). The formula calculated DPPH scavenging efficacy:
Whereas:
ODs: the absorbance of the sample at 517 nm wavelength.
ODc: the absorbance of the negative control at 517 nm wavelength.
Evaluation of antibacterial activity
The antibacterial activity of cephalothin and cephalothin@siRNP was determined by agar diffusion and minimum inhibitory concentration (MIC) assays. Briefly, a volume of 100 uL of bacterial culture ( ATCC 25922 or ATCC 23235™ ) on nutrient broth (NB) (containing 3 g meat extract, 5 g peptone, 5 g NaCl) and adjusted to 0.5 in MacFallen scale) was spread on a nutrient agar (NA) plate (containing 3 g meat extract, 5 g peptone, 5 g NaCl, 10 g agar). An amount of 50 uL of serial dilutions of cephalothin and cephalothin@siRNP was pumped to 6 mm wells on bacterial NA plates followed by incubation of 24 h at 37 °C. Namely, the cephalothin concentrations used to treat were from 1 to 10 µg/mL, while to treat were from 100 to 1000 µg/mL. Sizes of inhibition zones and reoccurrence of bacterial colonies were recorded11. The MIC was also determined using the same concentrations of cephalothin or cephalothin@siRNP on the NB medium for 24 h treatment followed by an absorbance measurement at 620 nm using Spectro–UV11 UV–Vis spectrometers (MRC Laboratory Instruments, Ltd, Israel).
Statistical analysis
All values were expressed as mean ± standard deviation (SD). The differences between the groups were examined for statistical significance using Student’s -test. A value of p < 0.05 was considered significant for all statistical analyses.
RESULTS
Characterization of siRNP and cephalothin@siRNP
We firstly evaluated the size and antioxidant activity of siRNP. The TEM image showed that siRNP presented a spherical shape (Figure 2A). By DLS measurements, the average size of siRNP was found at 43.83±1.95 nm and slightly increased to 50.15±0.78 nm after drug encapsulation (Figure 2B). The antioxidant activity of siRNP was evaluated via DPPH scavenging capacity with different concentrations from 10 to 200 µg/mL. As shown in Figure 2C, when siRNP concentration increased, the antioxidant activity of siRNP increased from 22.10% to 40.6%, compared to tempo from 53.01% to 93.04%. The results showed that siRNP had antioxidant activity and this activity was derived from the tempo radical attached in the core of nanoparticle.
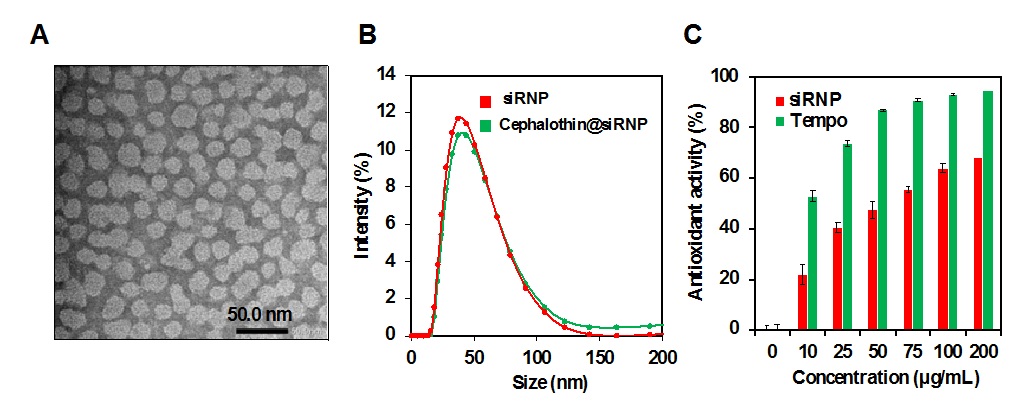
Characterization of siRNP.A) Transmission electron microscopy image of siRNP. B) Size distribution of siRNP and cephalothin@siRNP by dynamic light scattering (DLS) measurement. C) Antioxidant activity of siRNP by DPPH assay. Data express mean ± (SD), n=3.
We next evaluated the water solubility of cephalothin before and after encapsulation in nanoparticles. As shown in Figure 3, free cephalothin exhibited a partial solubility in water, which formed visible crystals precipitating in the bottom of the solution, suggesting the low bioavailability of these antibiotics for oral administration. When cephalothin was encapsulated into siRNP, the solution was clear and transparent, indicating that water solubility was significantly improved (Figure 3). These results suggested that siRNP can be utilized as a drug nanocarrier but also as an antioxidant agent, which may improve the therapeutic efficacy of the loaded antibiotics.

The solubility of cephalothin before and after encapsulation in siRNP.A) Solubility of cephalthin in water. B) cephalothin@siRNP. C) siRNP. Cephalothin in water exhibited low solubility with visible unsoluble particles, while cephalothin@siRNP was transparent in the aqueous solution.

The antibaterial acitivity of cephalothin and cephalothin@siRNP. A) Inhibition zones of cephalothin and cephalothin@siRNP on
In Figure 4A, inhibition zones of cephalothin and cephalothin@siRNP against and were determined by the agar diffusion method. The antibacterial activities of cephalothin and cephalothin@siRNP were plotted in Figure 4B, showing that cephalothin and cephalothin@siRNP performed antibacterial efficacy on both bacterial strains through large and clear inhibition zones on Petri plates. The diameter of inhibitory zone increased from 1.77 to 2.97 cm against and from 1.63 to 2.00 cm against (Figure 4B). The results showed that cephalothin's antibacterial activity was more effective against Gram-positive bacteria () than Gram-negative bacteria (). This is similar to previous studies from Hamilton-Miller 12 and Papich 13, which showed the antibacterial activity of cephalothin against Gram-positive bacteria was much higher than Gram-negative bacteria. However, statistical analysis on diameters of inhibition zones showed no statistically significant difference in antibacterial activities induced by cephalothin and cephalothin@siRNP (Figure 4B). To further investigate the antibacterial activity, we next evaluated the minimum inhibitory concentration (MIC) of cephalothin and cephalothin@siRNP against both bacteria. As shown in Figure 5A, there was no significant difference in the MIC of cephalothin and cephalothin@siRNP against ; however, we could observe a significant difference in the antibacterial activity of cephalothin@siRNP as compared to cephalothin in the strain (Figure 5B).

Minimum inhibitory concentration (MIC) of cephalothin and cephalothin@siRNP. (A)
Recurrence of in inhibition zones
To further investigate the antibacterial activity of cephalothin@siRNP against , we further extended the incubation time on the agar diffusion assay to observe the colony's formation.

Recurrence of
Regrowth of colonies was observed inside inhibitory zones of treated with cephalothin (Figure 6A). Interestingly, the number of regrowth colonies was significantly reduced in plates treated with cephalothin@siRNP in comparison to plates treated with cephalothin. Moreover, at a 250 and 500 µg/mL concentration, the number of regrowing colonies of treated with free cephalothin was significantly lower than that treated with cephalothin@siRNP (Figure 6B). Specifically, at a concentration of 250 µg/mL, after 30 h incubation, 76±7 of the regrowth colonies were observed in the cephalothin-treated plates, while only 27±11 colonies were found in cephalothin@siRNP-treated plates. Similarly at a 500 µg/mL concentration, quantilites of colonies of cephalothin@siRNP-treated sample was 35±11, which was 39.77% reduction in comparision with the cephalothin-treated sample (Figure 6B). At higher concentrations (750 and 1000 µg/mL), although the statistical difference between cephalothin and cephalothin@siRNP was not obtained, the lower number of CFU in cephalothin@siRNP was observed, indicating the siRNP contributed to the antibacterial feature of cephalothin to against the bacterial recurrence.
DISCUSSION
The current antibiotic drugs are insufficient therapy for the treatment of versatile infectious disease, and the development of new effective and safe drugs with high cost and time consuming are limited for applications. Extention of antibiotic effectiveness for treatment of bacterial infection is required. Nanotechnology has been applied to overcome antibiotic resistance in various bacterial strains since nanoparticulate drug carriers exhibit excellent physiochemical features, enhanced drug uptake, specific targeting. Various nanoparticles have been developed to fight against antibacterial resistance, including liposomes, solid lipid nanoparticles, polymeric micelles, silver nanoparticles14, 15, 16, 17. Current nanoparticles are merely designed as drug delivery systems and do not present the therapeutic effect. Low drug loading capacity and instability are other challenges of current nanocarriers 18. In this study, siRNP was developed and presented high stability, and high drug loading capacity to encapsulate a hydrophobic antibiotic agent (cephalothin) and investigated the antibacterial property. In fact, we previously confirmed that siRNP with ROS scavenging capacity is a promising nanocarrier for the delivery of hydrophobic anti-inflammatory and anti-cancer agents in the treatment of inflammation and cancer in mouse models7, 19. Here, cephalothin@siRNP significantly improved solubility of cephalothin with the size about several tens of nanometer along with antioxidant feature via DLS measurement and DPPH assay (Fig. 2 and 3).
Cephalothin is known to be an -lactam antibiotic that has strong coverage against Gram-positive strains, especially , and moderate effect against some Gram-negative bacilli 20. Regarding the antibacterial effect in this study, cephalothin encapsulated into siRNP exhibited antibacterial activity on both strains of and . Although there was no difference when compared with cephalothin in the agar diffusion method, cephalothin@siRNP still showed a slightly stronger effect, especially in the MIC result on ( Fig. 5B). To some extent, using siRNP encapsulated cephalothin, which improved the antimicrobial effect of cephalothin against Gram-negative bacteria. These are the initial results for the research about nanomaterials' applications in improving the efficiency of previous generations of antibiotics, when here are the current research trends 21, 22.
Recurrence of in inhibition zones by agar diffusion test is an exciting investigation of this study. The results showed that cephalothin@siRNP was more effective at inhibiting the reinfection of than cephalothin (Fig. 6), which may be assumed that the encapsulation of cephalothin by siRNP can slowly release and enhanced the effect of cephalothin together with the ROS scavenging effect of siRNP. In a previous study, LoïcLéger et al. demonstrated that β-lactam antibiotics increased ROS production in both Gram-positive bacteria and Gram-negative bacteria through the oxidation of membrane-associated demethylmenaquinone23. However, overproduction of ROS was reported to be one of the causes of antibiotic resistance in bacteria through gene mutation4. Hence, in this result, the ROS scavenging ability of nanoparticles maybe is a factor that prevents gene mutation of bacterial in drug resistance. These results demonstrated the potential of applying siRNP in treating antibiotic resistant bacteria.
CONCLUSIONS
In this study, cephalothin was successfully encapsulated by a silica-containing redox nanoparticle (siRNP) to form cephalothin@siRNP, which significantly improved the solubility of cephalothin. The antibacterial results showed that cephalothin@siRNP exhibited antibacterial activity against both Gram-positive strain and Gram-negative strain, but a significant improvement was obtained only in when comparing with the cephalothin-treated group. Interestingly, we found that cephalothin@siRNP significantly inhibited the recurrence of colonies after extending the incubation, suggesting the prolonged antibacterial activity of cephalothin@siRNP. Further investigations are being carried out to understand the underlying mechanism of this interesting phenomenon. Taken together, siRNP can improve the antibacterial effect and prevent MDR of low bioavailable antibiotics.
LIST OF ABBREVIATIONS
MDR multidrug resistance
siRNP silica-containing redox nanoparticle
PEG polyethylenglycol
siPMNT poly(silano aminomethylstyrene- - poly(4-(2,2,6,6-tetramethylpiperidine-1-oxyl) aminomethylstyrene)
ROS reactive oxygen species
DLS dynamic light scattering
DPPH 2,2-diphenyl-1-picryl-hydrazyl-hydrate
DMF dimethylformamide
NB nutrient broth
NA nutrient agar
COMPETING INTERESTS
The authors have no competing financial interests to declare.
Authors' Contributions
Tran N H, Pham H T have contributed in conducting experiments, collection of data and interpretation of data. Tran N H, Nguyen H T N have prepared and revised manuscript. Vong B L, Trinh N have supervised, prepared and gave final approval of the manuscript to be submitted.
ACKNOWLEDGEMENT
This work was supported by Vietnam National Foundation for Science and Technology Development (NAFOSTED under Grant number 108.05- 2017.327).

