Transfer hydrogenation of benzaldehyde over embedded copper nanoparticles
- 1. University of Science, Ho Chi Minh City, Vietnam
- 2. Vietnam National University, Ho Chi Minh City, Vietnam
Abstract
Transfer hydrogenation is one of the reactions of high industrial application and copper catalyst is widely used in variety of hydrgenated substrates. Unfortunately, these hydrogenated processes were usually performed at high temperature and pressure as well as high concentration of catalyst. In this study, we have tried to reduce the dangerous condition by using copper nanoparticles as catalyst and the catalytic activity will be evaluated via the transfer hydrogenation of benzaldehyde. Besides, copper nanoparticles were successful prepared by the reduction between CuSO4.5H2O and NaBH4 in the presence of PVP. All catalysts were fully characterized.
INTRODUCTION
Transfer hydrogenation has been studied since 18971, 2; it is still attracting the attention of many researchers by its convenient and powerful method to access a variety of industrial applications from organic synthesis to fine chemicals3, 4. Recently, many researches have been reported with high efficiency, excellent chemoselectivity, long-lived stability, and easy recovery when palladium5, 6, 7, 8 and nickel9, 10, 11 catalyst were used.
However, not many publications have been found in copper nanoparticles' uses; most of the reports focused on the hydrogenation of alkyl ketones12, 13, nitroarenes14, a polycyclic aromatic hydrocarbon15, 16, quinolines, alkynes, imines17 etc… Unfortunately, the reduced condition was usually carried out at a high temperature and dangerous atmosphere pressure. For example, W. Li and coworkers hydrogenated quinolines in high yield, up to 98% over Cu-AlO catalyst at 120 °C under 50 bar of hydrogen pressure within 24h18. Likewise, J. Wu . performed the hydrogenation of furfural at 150 °C under 4 Mpa hydrogen pressure within 3h, over 90% conversion was observed with CuNi-MgAlO as catalyst19. According to K. Suthagar, glycerol was hydrogenated over 15 wt% of Cu-SiO as a catalyst to obtain 1,2-propanediol in 95% conversion at 200 °C under 60 bar hydrogen pressure20.
Therefore, in an attempt to explore more the scope of catalytic processes available from embedded copper nanoparticles and reduce the high temperature, pressure, and amount of catalysts, we have tried to test the activity of the copper catalyst in a variety of organic synthesis reactions21. In which transfer hydrogenation is one of the reaction of high industrial application. Besides, immobilization of the metallic nanoparticles on solid materials has received a great interest because of their use in industrial applications, especially hydrogenation of carbonyl compounds. Though nanomaterials serve as an excellent heterogeneous catalyst, they often need additional support to acquire thermal stability. Therefore, varieties of materials like zeolites, aluminum oxides, aluminosilicates, silica gel, chitosan, activated carbon, zinc oxides, etc., have been used as supports nanocatalysts22, 23, 24. Among these materials, activated carbon, bentonites, aluminum oxide, zeolites, and zinc oxide are widely used as catalysts and support for the number of reactions.
This study focused on copper nanoparticles' preparation embedded on the supports such as bentonites, zeolites, activated carbon, zinc oxide, and aluminum oxide. Catalytic activity was evaluated via the transfer hydrogenation of benzaldehyde. The results will be presented in this report.
MATERIALS AND METHODS
Materials
Unless otherwise noted, all experiments were carried out in the air. Reagent grade copper (II) sulfate pentahydrate 98% (CuSO.5HO), aluminum oxide 99% (AlO), zinc oxide 99% (ZnO), benzaldehyde 99%, and sodium borohydride 98% (NaBH) were purchased from Merck (Germany). Potassium hydroxide 98%, zeolite (Zeolit), and polyvinyl pyrrolidone K-30 (PVP) were purchased from various Chinese suppliers (Xilong, China). Binh Thuan bentonite (Bent) and activated carbon were purchased from the local suppliers (Binh Thuan, Vietnam). Absolute ethanol and isopropanol were supplied by Chemsol (Ho Chi Minh City, Vietnam) and used as received.
Characterization
The morphology of catalysts was examined by scanning electron microscope (SEM, Hitachi S4800, Japan). Transmission Electron Microscopy (TEM) images were collected using FEI Tecnai G2 F20 (University of Technology, Ho Chi Minh City). The X-ray diffraction (XRD) data of all samples was collected in a Bruker D8 powder X-Ray (Vietnam Petroleum Institute, Ho Chi Minh City) with Cu Kα radiation running at 35 kV/30 mA in the 2θ range 5¸75 with a step size of 0.2/min. Nitrogen adsorption-desorption isotherms were collected at 77K using Brunauer–Emmett–Teller calculation (AUTOSORB-1C Quantachrome, INOMAR center, VNU-HCM), all the samples were degassed at 100 C and 10 Pa. Atomic absorption pectroscopy (AAS) was analyzed on Agilent 240AAS (Laboratory of Analysis-University of Science, Ho Chi Minh City). GC-MS was obtained using an Agilent 7890A series model with an electron energy of 20 or 70 eV (Laboratory of Natural compound, University of Science, Ho Chi Minh City). All the catalytic test were performed in Multireactors Carousel 12 plus (Laboratory of Catalysis- University of Science, Ho Chi Minh City).
Catalyst preparation
To the 250 mL two-necked round bottom flask, 0.4 g of PVP and 70 mL of deionized water (DI) were added. After stirring for 15 min, 0.50 g of CuSO.5HO (2.0 mmol) was dissolved in the mixture at 80 C. In another flask, 0.15 g of NaBH in 50 mL of DI water was prepared. Then, the solution of the reducing agent was dropwise added to the mixture of copper salt. The mixture was stirred for 6h until the black solution appeared.
The copper nanoparticles were then loaded into the supports X (X = Bent, C, Zeolit, ZnO, and AlO which were calcinated at 120 C in 8h) in a suitable amount by low-pressure method at room temperature. This process was repeated several times to make sure all the copper nanoparticles were fully loaded into the supports. The obtained powders were dried at 80 C under vacuum for 5h.
Catalyst evaluation
In this work, the catalytic activity of copper nanocatalysts was investigated via the hydrogenation of benzaldehyde under the liquid phase in the presence of potassium hydroxide. The catalytic evaluation of Cu-X was carried out in 20 mL multi reactor with stirring at 60 C under reflux condensation. In this process, 5.0 mol% of Cu-X was used to hydrogenate benzaldehyde (5.0 mmol), isopropanol (IPA, 5.0 mL), and 1.0 mL of potassium hydroxide solution 5% in isopropanol. Hydrogen was directly connected through Schlenk line to the reaction at atmospheric pressure within 60 min. The conversion of substrate and selectivity of products were analyzed by GC and GC-MS (HP5 column 30 m x 0.25 mm, FID detector). Reproducibility was checked by repeating the measurement several times and was found to be within acceptable limit.
RESULTS
Copper nanoparticles were simply synthesized by the reduction of copper sulfate pentahydrate using sodium borohydride as reduction agents. The mixture of nanoparticles was then loaded into supports X with various concentrations, as seen in

XRD patterns of a) Cu-Bent; b) Cu-C; c) Cu-Zeolit; d) Cu-ZnO; and e) Cu-Al2O3. All were dried at 60 °C under vacuum for 8h without further calcination.
Besides, as shown in Figure 1 two reflection peaks centered at 2θ of 43.4° and 50.2° are assigned for Cu, indexed to the (111) and (200) plane of copper (JCPDS 004−0836). This confirmed the reduction of copper salt to metallic copper. Even though the peaks are quite weak because of the low concentration of metal particles in the samples. Furthermore, the corresponding diffraction peaks of ZnO, Zeolit and AlO located at the position of 2θ = 29.95°; 34.62°; 36.49°; 47.75°; 56.72°; 63.01°, 21.90°; 24.21°; 27.43°; 30.25°; 33.21°; 34.53°; 36.17°; 45.50°; and 25.90°; 35.43°; 38.04°; 43.61°; 52.92°; 57.77°; 66.80°; 68.42°, re pectively. Meanwhile, C and Bent are amorphous lattice structures leading to the XRD patterns as the noise at the baseline (Figure 1a and Figure 1b).

TEM images of a) Cu nanoparticles taken at 50 nm; b) Cu-Zeolit taken at 20 nm.
On the other hand, TEM images of copper nanoparticles in Figure 2a showed that these nanoparticles had a spherical morphology with an average particle size in the range of 14¸16 nm. Moreover, Zeolit supported Cu nanoparticles in Figure 2b described that almost all the copper nanoparticles were well dispersed on Zeolit. Whereas SEM defined the morphology surface of catalysts; in fact, in Figure 3a, the surface of Cu-Bent was occupied by slit-shaped pores. Meanwhile, in Figure 3b-e the spherical shapes of Cu were attached on the surface and inside the supports' pores.

SEM images of a) Cu-Bent; b) Cu-C; c) Cu-Zeolit; d) Cu-ZnO; e) Cu-Al2O3. The images were taken at 500 nm and 1.0
DISCUSSION
Copper nanoparticles were usually synthesized by the reduction of copper salt using different methods. In reality, J. Wu and coworkers performed via the hydrothermal reduction at high temperature in order to reduce copper nitrate to metallic copper under hydrogen flow12. Likewise, L. Lin . carried out reducing copper nitrate at 500 °C by tetramethylammonium hydroxide as a reducing agent25. R. Beerthuis and coworkers used the incipient wetness impregnation method to reduce copper precursors following to calcinate at high temperature13. In sum, all these methods required high temperature and long time calcinated, leading to low yield and danger for the handle. Therefore, in this study, we prepared the copper nanoparticles by reducing sodium borohydride at RT, following in situ loaded into supports X. The XRD patterns in Figure 1 indicated that all the copper ions were reduced to metallic copper. On the other hand, in the presence of PVP, copper nanoparticles were more stable, it could be explained in terms of the distribution of copper nanoparticles in the PVP solution, which is a well-known polymer with a large molecular size and free-electron couple on nitrogen site that can bond with copper nanoparticles. Hence PVP acts as a protecting agent to avoid the agglutination and deposition of copper nanoparticles.
Specific surface area and AAS analysis of catalysts
|
Entry |
Catalysts |
SBET (m2.g-1) | ||||
|
|
|
Bent |
C |
Zeolit |
ZnO |
Al2O3 |
|
1 |
Blank |
54.08 |
318.36 |
64.78 |
48.75 |
16.99 |
|
2 |
Cu |
49.50 |
107.70 |
31.36 |
55.30 |
6.40 |
|
AAS (%Cu) |
6.28 |
8.61 |
7.39 |
7.36 |
2.80 | |
Besides,
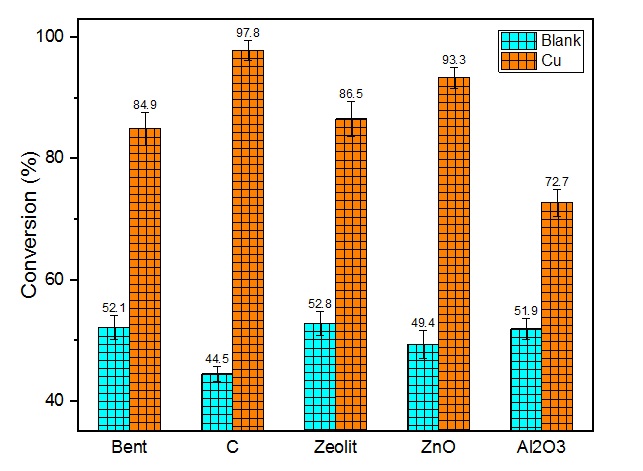
Conversion of transfer hydrogenation of benzaldehyde over Cu-X catalysts (X = Bent, C, Zeolit, ZnO, and Al2O3). Reaction condition: 5 mol% of catalyst was used at 60 °C within 1h.
To evaluate the efficiency of the catalysts, the transfer hydrogenation of benzaldehyde was performed in the presence of potassium hydroxide in isopropanol within 60 min. All the experiments were carried out at 60 °C and summarized in Figure 4. In which the conversion of benzaldehyde was up to 97.8% in the case of Cu-C catalyst, it could be explained in terms of the specific surface area as well as the AAS analysis of Cu-C, namely surface area of Cu-C was over 107 mg and the highest concentration of Cu on supported C was 8.61%. Likewise, the Cu-ZnO catalyst gave the high activity in the transfer hydrogenation of benzaldehyde as well, 93.3% conversion was obtained within 60 min. However, in the case of Cu-AlO, the conversion of benzaldehyde slightly decreased to 72.7% because of the low concentration of copper particles in AlO at 2.8%, as seen in Figure 5. Even though the parent supports alone possessed the moderate conversion. These were confirmed that copper catalysts were active in the transfer hydrogenation of carbonyl substrates under mild conditions even though the low concentration of copper in the catalyst.
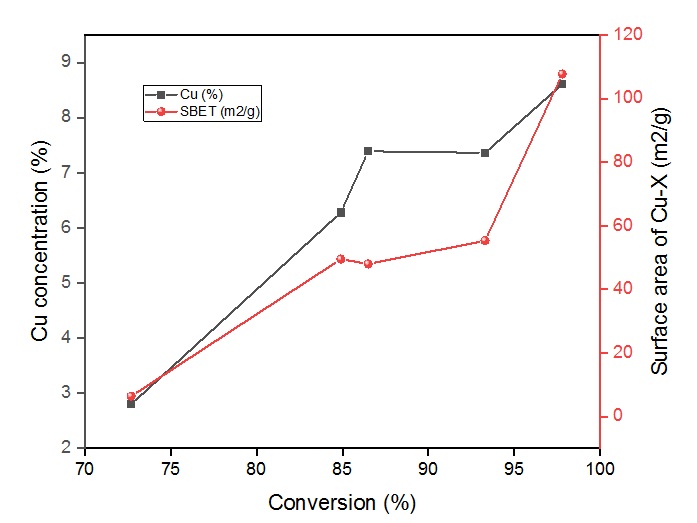
Influence of Cu concentration and surface area of catalysts on the conversion of benzaldehyde.
CONCLUSION
This study demonstrated that copper nanoparticles are the powerful catalysts for the transfer hydrogenation of carbonyl substrates at low temperatures. Namely, the conversion was up to 97.8% within 60 min in the hydrogenation of benzaldehyde in activated carbon-supported copper nanoparticles. Besides, all the copper catalysts were characterized in detail, in which the size of copper nanoparticles is around 14-16 nm, and all copper ions were reduced to metallic ones.
ABBREVIATIONS
Bent: bentonites
DI: deionized
FID: flame ionization detector
PVP: polyvinyl pyrrolidone-K30
Zeolit: zeolites
COMPETING INTERESTS
The author (s) declare that there are no conflicts of interest regarding the publication of this paper.
ACKNOWLEDGMENT
This research is funded by the Graduate University of Science and Technology under grant number GUST.STS.ĐT2020- HH09. The author especially thanks to University of Science - Ho Chi Minh City for technical support.

