Fractionation of lignin produced from the Earleaf Acacia tree by sequential industrial organic solvents
- School of Biotechnology, International University, Ho Chi Minh City, Vietnam
- Industrial Development Center of Southern Vietnam, Ministry of Industry and Trade, 12 Nguyen Thi Minh Khai street, District 1, Ho Chi Minh City, Vietnam
- Faculty of Chemical Engineering, Ho Chi Minh City University of Food Industry, 140 Le Trong Tan Street, Tay Thanh Ward, Tan Phu District, Ho Chi Minh City, Vietnam
- Institute of Research and Development, Duy Tan University, Da Nang City 550000, Vietnam
- Faculty of Chemical Engineering, Industrial University of Ho Chi Minh City, Ho Chi Minh city, Vietnam, 12 Nguyen Van Bao Road, Ward 4, Go Vap District, Ho Chi Minh City, Vietnam
Abstract
Introduction: Understanding the fractions of lignin is important for further conversion of lignin into valuable products. Herein, the “home-made” lignin from Earleaf Acacia tree was extracted by sequential industrial organic solvent and characterized each fraction to reveal its properties for further catalytic applications.
Methods: In this work, lignin was prepared from the Earleaf Acacia tree using the soda method. Then, the prepared lignin was fractionated by sequential solvents of ethyl acetate, ethanol, methanol, and acetone. Each lignin fractions were characterized by FT-IR and GPC.
Results: The FT-IR results confirmed the soda method can produce lignin from woodchips. The fractionation of lignin separated the lignin mixture into different molecular weight fraction from light – medium into heavy compounds.
Conclusion: Lignin was produced from woodchips using the soda method successfully. The fractionation using the sequential organic solvents showed the separation of different molecular weight of lignin, which allow to apply for the further conversion into useful products.
Introduction
Lignin is the main component of vascular plants, along with cellulose and hemicellulose. Therefore, it has a huge abundant resource on the earth, and it contains ca. 20-30% of lignocellulosic biomass 1. Currently, lignin is mainly produced from the pulp and paper industry and is considered a solid waste for burning to produce heat and energy 2. However, lignin has a high potential application in the industry due to its polymer structure 3, 4, 5 and aromatic backbones 6, 7, 8. Indeed, lignin is a biopolymer with C—O—C and C—C linkages of the phenylpropane unit, which contains hydroxyphenyl (H), guaiacyl (G), and syringyl (S) types (Figure 1) 9.
Due to this structure, lignin can be applied in cement, binders 10, surfactant 11, and friendly biopolymer with biodegradability, antioxidants, and UV-protection 12. In addition, the conversion of aromatic backbones to form aromatic compounds using for many applications, such as automotive brakes, wood panel products, surfactants, phenolic resins, phenolic foams, dispersants, polyurethane foams, and epoxy resins 7, 13, 14. In parallel, the demand for green fuels increases due to the development of the industry and the reduction of the dependence on fossil resources. Currently, many studies are focusing on the conversion of lignin model compounds and industrial lignin 15, 16, 17, 18. However, lignin is a complex mixture of many polymers, so that the transformation of “full” lignin is hard to be selective and control the desired products. To make an easy way to convert lignin, lignin is fractionated into different fractions using industrial organic solvents. The conversion of real lignin faced several problems, such as challenging reaction conditions 19, 20 and the fast deactivation of the catalyst due to the ester structures in lignin 21. Indeed, the conversion of the lignin’s fractions is easier than real lignin 22. Therefore, lignin's fractionation is very interested in recent years 23, 24, in which lignin can be fractionated into different fractions with different molecular weights 23, 24, 25. The lignin’s fractions with different molecular weights can be applied for different purposes and transformed into chemicals and fuels with a suitable condition. Hence, the fractionation of a complex mixture of lignin is important for further studies.
To fractionate lignin into different parts, lignin produced from the Earleaf acacia tree, an abundant tree to reforest and use in the pulp and paper industry in Vietnam, is fractionated into different fractions. The solvent extraction has been applied to fractionate according to molecular weight by sequential extraction with organic solvents 26, 27, 28, 29, 30. Duval et al. 3 showed that the trend of the yields of the soluble fraction and average molecular weight and polydispersity of the soluble fraction in the different solvents is acetone > methanol > ethanol > ethyl acetate. Based on that result, this solvent extract sequence is utilized for the prepared lignin. FT-IR and GPC techniques used characterize each fraction to study the structure of the fraction and provide a full picture of the “small” lignin fractions for further conversion of lignin in the next step.

The structure of lignin: (a) A model structure, and (b) the main units and linkages of lignin. Reproduced from Open-access ref.
Materials and Methods
Materials
The Earleaf acacia woodchips were purchased in Bien Hoa City, Dong Nai province then were cut into small pieces with a dimension of 30 mm x 60 mm. Ethyl acetate (Fisher, 99.8%), Methanol (Fisher, 99.9%), Acetone (Fisher, 99.8%), Ethanol (Fisher, 99.8%), NaOH (Fisher, extra pure), HCl (Fisher, 37%), and Dimethyl sulfoxide (Fisher, 99.9%) were used without any pretreatment. Lignosulfonic acid sodium salt (denoted as lignosulfonate) purchased from Sigma-Aldrich was used as a reference of lignin.
Lignin preparation and fractionation
The Earleaf acacia woodchips were dried at 105C until unchanged weight; the percentage of water in the wood is 11.64%. The dried wood, NaOH, and water were filled into a batch reactor with the ratio of dried wood/ NaO/ HO = 500 g/ 90 g/ 2500 mL, then processed as described in Figure 2. Firstly, the batch reactor was heated at 100C to reach the pressure of 40 bar, then released the pressure to atmospheric pressure. Next, the system was closed and heated to 160C and hold for 5h; the pressure at this point was ca. 70 bar. After finishing the pulping process, the system was released the pressure and collected pulp and black liquid. A gel of lignin was formed by neutralizing the black liquid with HCl 1M at pH = 6 ÷ 7. The dried lignin (denoted as lignin_prepared) was collected after freeze-drying lignin gel at −50C for 12h, then kept in the dark color bottle and stored in a freeze before using it.

The scheme of lignin production from wood chips of the Earleaf Acacia tree using NaOH at 160 °C for 5h followed by neutralizing using HCl 1M and freeze-drying at – 50 °C for 12 h.
For fractionation, the prepared lignin was sequentially fractionated with ethyl acetate, ethanol, methanol, and acetone as described elsewhere (3). The lignin and specified solvent with a ratio of 1 g :10 mL of lignin/solvent were added in a 100 mL beaker. After that, the mixture was agitated for 1h using a magnetic stirrer at room temperature. The undissolved material was filtered over a P5 Qualitative filter paper with particle retention of 5-10 µm (Fisher Scientific, Hampton, NH). The solid fraction was dried at 50°C for 30 minutes to remove the remaining solvent, and then it was fractionated with a sequential solvent, as illustrated in Figure 3. The undissolved solid after each solvent extraction was denoted as lignin_solvent, such as lignin_EA, lignin_EtOH, lignin_MeOH, and lignin_Ace.

The scheme of fractionation of lignin using the sequential industrial organic solvents of ethyl acetate, ethanol, methanol and acetone with a ratio of solid/solvent = 1 g/10 mL at room temperature.
Characterization
FT-IR study
FT-IR spectra of the prepared lignin and fractions were recorded in the air using a Jasco spectrometer equipped with an ATR cell (attenuated total reflectance). The IR spectra were performed with 16 scans, scanning speed of 2 mm/s at the resolution of 4 cm and a wavenumber range between 4000 cm to 550 cm.
GPC study
Lignin solutions, 2 mg/mL, were prepared in Dimethyl sulfoxide (DMSO). The polydispersity of dissolved lignin was determined by gel permeation chromatography (GPC) using an Agilent 1100 – GPC with a differential refractive index detector (RID detector). The separation was achieved by a PLgel Mix A column at 40 °C using DMSO as the mobile phase at a flow rate of 0.5 mL/min. Polystyrene standards were used for calibration. The GPC measurement used to determine molecular mass using linear polystyrene as the reference material in this study is only valid for the relative molecular mass distribution of lignin extracted with different organic solvents. The molecular mass value of lignin is not considered to be exact.
Results
Lignin production
In this work, lignin was produced via the traditional soda process. HCl neutralized liquid lignin solution to pH = 6 – 7, then freeze-dried to obtain lignin powder. The yield of lignin, in this case, was around 13.54 wt.%. This yield is not high compared to the lignin content (20 – 30%) in lignocellulosic materials. However, it is in agreement with the previous study proving that lignin's yield strongly depends on the value of pH treatment [24].
Lignin fractionation
Lignin was successfully fractionated using four industrial solvents, as showed in Figure 3. The highest yield of dissolved lignin in this process was obtained using ethanol solvent following by ethyl acetate, methanol, and acetone. However, any following step is an accumulation of the previous step. The results also figured out that all four solvents did not dissolve all lignin, and it remained ca. 30% residue after fractionation process. Only 6.7% of dissolved lignin was extracted using acetone at the final step due to a high amount of long-chain polymer remaining in the lignin sample. Suggesting that the fractionation process of the prepared lignin can be carried out using three first solvents to fractionated into four fractions (three dissolved lignin fractions and one solid residue) with a high percentage (> 20%) for further conversion of each lignin fraction.
FT-IR study
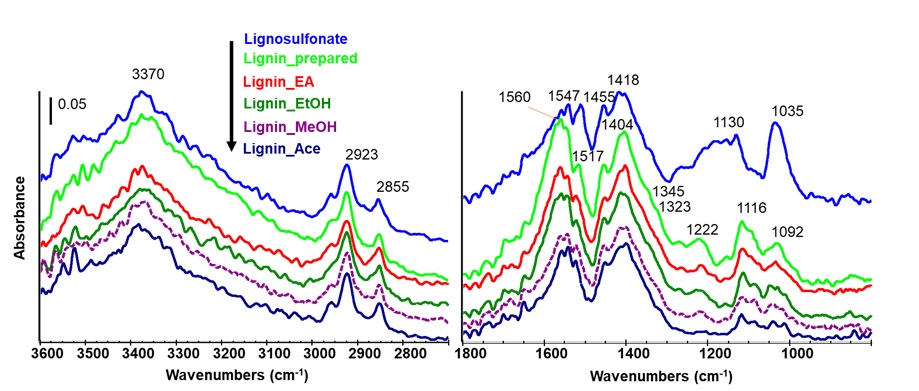
The ATR-FT-IR spectra of the prepared lignin, solid fractions after fractionating using the sequential industrial organic solvents of ethyl acetate, ethanol, methanol and acetone, and a reference, lignosulfonate.
It can be seen that Figure 4 showed the typical lignin spectra of the prepared lignin and fractions 22, 31, 32. The peaks of the prepared lignin and its fractions are similar to the lignosulfonate. For the prepared lignin and its fractions, the peak at ca. 3370 cm was assigned to OH stretching, indicating that all lignin samples contain a large number of hydroxyl groups. The peaks at 2923 and 2855 cm were assigned for −CH and −CH stretching. The absorption peaks in the range of 1560 – 1404 cm were assigned to the skeletal vibrations and C−H deformation combined with aromatic ring vibrations 31. The peaks at 1345, 1323, 1222, and 1116 cm indicated that the prepared lignin is mainly composed of G-type and S-type units 22. In comparison, the absorption peak at 1092 cm corresponded to the deformation vibration of aromatic C−H in-plane 31. Comparing the intensity between the lignin’s fractions and the prepared lignin, the peaks of aromatic ring vibrations in the range of 1560 – 1404 cm decreased the intensity through extraction with a sequence of organic solvents. Also, the peaks in the range of 1345 – 1116 cm corresponding to G type and S type units decreased the intensity and almost disappeared the peak at 1222 cm in the case solid after fractionating with acetone, suggesting that the sequential organic solvents used extracted different fractions of lignin very well.
GPC study
The GPC data showed how effective fractionation using industrial solvents. Our data are comparable with the literature19, 25, 33. The prepared lignin showed the long-range distribution of molecular weight (MW) with most low molecular weight (Figure 5). After fractionating using ethyl acetate, the low molecular weight compounds seemed to dissolve into the ethyl acetate solution resulting in the broad curve in MW from 1000 to 7000 Da. The next step with ethanol presented a clear distribution of remained lignin solid with the major medium molecular weight fraction. Besides, the low molecular weight fraction and likely a part of the medium molecular weight fraction were dissolved into ethanol solvent. For the methanol and acetone solvents, molecular weight distribution is almost similar due to the low extraction using acetone. The high molecular weight fractions are dominant in both cases, suggesting that both methanol and acetone dissolve very well the medium and a part of high molecular weight fractions. For acetone solvent, the curve is slightly different from that of methanol. The 6.7% extracted fraction through acetone changed the shape of molecular weight distribution of the remaining solid lignin, suggesting that methanol and acetone can dissolve a high amount of the medium and high molecular weight fractions.

GPC curves of the prepared lignin and solid fractions after fractionating using the sequential industrial organic solvents of ethyl acetate, ethanol, methanol and acetone.
Discussion
The ATR-FT-IR spectra confirmed lignin produced from the Earleaf Acacia tree is successful. The IR spectra showed similarities with the lignin from Sigma-Aldrich as a reference. However, the yield is lower than expected, suggesting that the improvement needs to study to enhance the yield of lignin from woodchips.
For the fractionation, the data from fractionation and GPC confirmed the efficiency of using the sequential industrial solvents, including ethyl acetate, ethanol, methanol, and acetone. The GPC showed that the light compounds come out first with ethyl acetate following by heavy compounds with the next solvents. GPC data allow us to decide the way to separate the mixture of lignin for further experiment. Moreover, GPC data and fractionation indicate that acetone's final extraction is not necessary due to the low yield of this fraction and similar GPC profile with the solid via methanol extraction. Perhaps the performance of acetone is quite similar to methanol. In short, the fractionation of lignin with the sequential industrial solvents can separate lignin into different molecular weight parts and tailor the fraction of lignin for further conversion. The well-extraction of heavy compounds using methanol and acetone suggests that these solvents may be applied for the conversion of lignin, such as in the catalytic conversion of lignin.
Conclusions
We can conclude that the soda process of transforming woodchips into lignin is successful. The sequential industrial solvents fractionated lignin into different molecular weight fractions from light and medium to heavy compounds confirmed by GPC data. The fractionation using organic solvents is necessary to separate the mixture of complex compounds into different small fractions, which are easy to convert into desired products.
LIST OF ABBREVIATIONS
Ace: Acetone
ATR: Attenuated total reflectance
DMSO: Dimethyl sulfoxide
EA: Ethyl acetate
EtOH: Ethanol
FT-IR: Fourier-transform infrared spectroscopy
GPC: Gel permeation chromatography
MeOH: Methanol
RID: Refractive index detector
COMPETING INTERESTS
The author(s) declare that they have no competing interests.
ACKNOWLEDGMENT
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.05-2019.39.

