The effect of excitation light source and humidity to photocatalytic activity of g-C3N4 nanosheets for NO removal
- Faculty of Materials Science and Technology, University of Science, VNU-HCM, 227 Nguyen Van Cu Street, District 5, Ho Chi Minh City, 700000, Vietnam
- Vietnam National University-Ho Chi Minh City, Linh Trung Ward, Thu Duc District, Ho Chi Minh City, 700000, Vietnam
Abstract
Introduction: Photocatalysis using nanostructured semiconductors is the potential strategy to solve the problem of environmental pollution. Besides traditional semiconductor materials, the novel polymeric metal-free semiconductor g-C3N4 has emerged as a potential substitute material because of its many outstanding features.
Methods: This study successfully synthesized two dimensions (2D)-structured g-C3N4 nanosheets by a simple thermal-exfoliation method with annealing route at 2 oC/min. Firstly, melamine was placed in a ceramic crucible with cover and then undergone the annealing route at 550 oC for 2 h to develop into the g-C3N4 bulk. Then the assynthesized g-C3N4 bulk was further annealed without the cover at 550 oC for 2 h to form the final product, g-C3N4 nanosheets.
Results: The results of XRD patterns and FTIR spectra show two typical diffractions peaks and chemical bonds that characterize the g-C3N4 matrix. The TEM images demonstrated that the as-prepared g-C3N4 possesses 2D-structured material, including several singly exfoliated sheets with a width of around several hundred nanometers. The photocatalytic NO removal efficiency of g-C3N4 nanosheets is highest at 48.27% under 30 min solar irradiation at 70% humidity. Meanwhile, the NO2 conversion yield is very low, only 9.44%, much smaller than the NO decomposition efficiency to form NO3 ? ion products. The results of trapping tests indicated that the hole plays the most critical role in the photocatalytic process of g-C3N4 nanosheets. Especially, the photocatalytic NO removal efficiency still achieves 45.03% after the recycling test. Moreover, all characteristic peaks and chemical bonds in material remain even undergoing fifth times reuse as the results of XRD and FTIR.
Conclusion: From various modern analytic characterization methods and photocatalytic investigation, we can concluse that g-C3N4 nanosheets are very stable and possible to apply in practical applications to decompose NO gas at atmospheric conditions.
INTRODUCTION
In recent years, the accelerating pace of industrialization, modernization, and population explosion has harmed the environment due to the emissions of greenhouse gases such as NO, NO, SO, CO, CO from means of transportation, industrial zones, factories, etc. 1. Among them, NO is one of the most toxic gases emitted mainly from vehicles and partly from natural phenomena such as volcanic explosions, thunderstorms2, 3. Moreover, the high concentration of NO gas in the atmosphere will negatively impact the environment causing smog formation, photochemical smog, and reaction with O to puncture the ozone layer 4. Especially, NO gas will directly affect human health, causing diseases of the liver, respiratory system, blood vessels, etc. 1, 4, 5.
To solve this problem, many research groups worldwide have spent a lot of effort in researching to remove NO gas from the air by methods such as electrostatic air purification, chemical oxidation, wet collectors, physical treatment, biological, and photocatalysis 5. Therein, the photocatalytic method using the nanostructured semiconductor material has emerged rapidly as an inexpensive, efficient, and environmentally friendly method that can decompose contaminants at high concentrations5, 6, 7. For instance, TiO, ZnO, SnO are traditional semiconductors that have been widely studied in the photocatalytic field for many decades. Still, most of them are limited by a few factors such as large bandgap, small specific surface area, complicated synthesis method that have restricted the applicability of the photocatalytic method 5, 8, 9. Recently, the novel material graphitic carbon nitride (g-CN) has been widely studied and known as a polymeric metal-free semiconductor for use in photocatalytic fields such as wastewater treatment, water-splitting, and pollutant gas treatment 10. Thanks to the superior properties of the large specific surface area and narrow bandgap of about 2.7 eV, the photocatalytic ability of the g-CN is significantly improved10. Especially, the morphology of g-CN in 2D-structured nanosheets can optimize the effective area of the photocatalyst that facilitates the reactions between the incident light and the surface of the photocatalyst. In addition, the fabrication of 2D material is feasible because the C-N bond to form g-CN sheets is the covalent bond that is much stronger than the van der Waals weak physical bond between the sheets10, 11. There are many ways to synthesize g-CN nanosheets, such as physical method, chemical method with acid or base solvents, ultrasonic exfoliation, etc. However, most of these methods require expensive facilities and are not environmentally friendly because of using acid and base solvents11. To overcome this problem, the thermal-exfoliation process is an optimal strategy to form g-CN nanosheets while ensuring environmental safety factors.
In this study, we synthesize g-CN nanosheets by a simple thermal-exfoliation method. First, these modern analytical methods, such as X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, transmission electron microscopes (TEM), and UV-Vis diffuse reflectance spectroscopy (DRS) are used to investigate the properties of the material. Then, the g-CN nanosheets are evaluated for the photocatalytic NO removal ability at 500 ppb (part per billion) of concentration under various conditions of irradiation and humidity to find the optimal condition to evaluate the applicability in Vietnam. In addition, the trapping tests are conducted to elucidate the main factor that contributes to the photocatalytic process of g-CN nanosheets. Moreover, we also conduct recycling experiments to evaluate the practical applicability of the materials. Finally, a photocatalytic mechanism based on the g-CN nanosheets is proposed and described concretely from the results obtained.
Materials-Methods
Chemicals and materials
All chemicals were analytical grade reagents and used as received without further purification. Melamine (CHN, 99.99%), potassium iodide (KI, 99.99%), potassium dichromate (KCrO, 99.99%), p-benzoquinone (BQ, CHO, 99.99%), and deionized water (DI) is extracted from the Puris Evo-UP Water System.
The synthesis procedure of the materials
The g-CN nanosheets were fabricated by the straightforward thermal-exfoliation method. Detailly, 1 g melamine was placed in a ceramic crucible with cover and then undergone the annealing route at 550 C for 2 h to develop into the g-CN bulk. Then the as-synthesized g-CN bulk was further annealed without the cover at 550 C for 2 h to form the final product, g-CN nanosheets. The ramping rate of the annealing route is 2 C/min. The whole material synthesis process was illustrated in detail, as shown in Figure 1.

The synthesis process of g-C3N4 nanosheets through a straightforward thermal-exfoliation method by using melamine as the precursor.
Characterizations of materials
The various modern analytical techniques have been applied, such as X-ray diffraction (XRD, using a Bruker D8, Advance 5005 with Cu Kα radiation (k = 0.154064 nm)), Fourier transforms infrared (FTIR) spectroscopy (Jasco V- 4700 spectrometer), transmission electron microscopes (TEM, JEM 2100, JEOL, Japan), and UV-Vis diffuse reflectance spectroscopy (DRS, JASCO-V550) to investigate the properties of materials. Therein, phase composition, chemical bonds, morphology, and optical properties of the materials are surveyed by XRD, FTIR, TEM, and DRS, respectively. Furthermore, for evaluating the photocatalytic activity of g-CN nanosheets for NO removal, we conducted the measurement procedure as our previous study under different excitation light sources listed as shown in
Detailed parameters of the excitation light sources
|
Notation of exciting light sources |
Manufacturer |
Power (W) |
Wavelength (nm) |
|
Solar-OSRAM |
OSRAM, Germany |
300 |
280 – 750 |
|
Vis-OSRAM |
OSRAM, Germany |
300 |
380 – 750 |
|
Solar-ABET |
ABET, USA |
150 |
300 – 750 |
|
UV-OSRAM |
OSRAM, Germany |
18 |
254 |
RESULTS
Figure 2a clearly shows two strong peaks located at 13.2 and 27.4of the g-CN nanosheets pattern. In Figure 2b, FTIR spectra reveal a marked change in the region from 1700 to 500 cm after undergoing a thermal-exfoliation process of melamine precursor. TEM images (Figure 3a-b) with different magnifications showed that the morphology of the g-CN is a 2D-structured material including several singly exfoliated sheets with a width of around several hundred nanometers. In addition, we further measure and compare the bandgap of g-CN material using the UV-Vis DRS method.Figure 4 indicated that the bandgap of g-CN bulk and g-CN nanosheets is 2.65 eV and 2.77 eV, respectively. The results clearly show that g-CN in nanosheets form has a wider bandgap than g-CN in bulk form.

X-ray diffraction (XRD) pattern (a) and Fourier transforms infrared spectroscopy (FTIR) spectrum (b) of g-C3N4 nanosheets.

Transmission electron microscopes (TEM) images of g-C3N4 nanosheets at 200 nm (a) and 100 nm scale (b) in the same shooting position.

UV-Vis diffuse reflectance spectroscopy (DRS) spectra (a) and Tauc plots (b) of g-C3N4 bulk and g-C3N4 nanosheets.
Figure 5a shows the results of the photocatalytic activity of g-CN nanosheets for NO removal under various excitation light sources at a humidity of 70%, consistent with the actual conditions in Vietnam. The results showed that the photocatalytic NO removal efficiency of g-CN nanosheets is the highest at 48.27% for the case of Solar-OSRAM. Meanwhile, the photocatalytic NO removal efficiency of the remaining cases is very low at 37.52%, 8.27%, and 7.26% for Vis-OSRAM, Solar-ABET, and UV-OSRAM, respectively. In addition, the NO conversion yield of the Solar-OSRAM case is very low at 9.44%, which is approximately 4.1 times smaller than the NO removal efficiency to green product (NO). Meanwhile, the conversion yield of other cases is very high compared to NO removal to NO(Figure 5b). Moreover, we tabulated key photocatalytic parameters to compare more visually and clearly with recent studies in Table 2. As a result, we can easily see that the performance of g-CN nanosheets with a single component is quite superior to that of the material combinations previously studied for NO degradation. After identifying that the photocatalytic efficiency of g-CN nanosheets is the highest under the solar irradiation, we continue to investigate how the humidity in the atmosphere will affect the photocatalytic process under the solar irradiation, as shown in Figure 5c. The results showed that after reducing humidity to 40% and 20%, the photocatalytic efficiency was decreased linearly by 37.52% and 34.18%, respectively. Besides, the NO conversion yield was also increased significantly by 11.74% and 13.37%, respectively (Figure 5d).

Photocatalytic NO removal efficiency (a), NO removal efficiency, and NO2 conversion yield (b) in different excitation light sources at 70% humidity, photocatalytic NO removal efficiency (c), NO removal efficiency, and NO2 conversion yield (d) in different humidity conditions under solar irradiation of g-C3N4 nanosheets.
A comparison of photocatalysts for the removal of NO
|
Photocatalyst |
NO conc. (ppb) |
Exciting light sources |
Synthesis method |
Efficiency (%) |
Ref. |
|
SnO2/PANI |
500 |
OSRAM-300 W |
Hydrothermal |
14.43 |
|
|
C-N-S-TiO2 |
400 |
Halogen lamp-300W |
Hydrothermal |
25 |
|
|
Ag/ZnO |
400 |
Xenon-300W |
Microwave-assisted one-pot |
55 |
|
|
g-C3N4 |
500 |
OSRAM-300 W |
thermal-exfoliation |
48.27 |
This study |
To identify the main factor in the photocatalytic process, we conducted photocatalytic trapping tests under solar irradiation conditions at 70% humidity with the presence of KI, KCrO, and BQ to trap factors h, e, and O, respectively (Figure 6a). The photocatalytic NO removal efficiency was significantly declined by 4.56%, 17.31%, and 35.09% in the existence of KI, KCrO and BQ, respectively. In addition, we also calculate the reaction kinetic k value to investigate the photocatalytic activity of g-CN nanosheets quantitatively. As expected, the results of Figure 6b indicate that the k value of the addition of KI is 0.004 min, which is much lower than another case (0.123 min/pure g-CN nanosheets; 0.062 min/g-CN + KCrO; 0.101 min/ g-CN + BQ). Moreover, we also investigated the material's durability for practical application by conducting a reusable experiment 5 times of photocatalyst. Figure 6c shows that the photocatalytic NO removal efficiency of the g-CN nanosheets is still very high at 45.05% after recycling tests. Figure 6d presents that the NO conversion yield increases linearly from 9.44% to 16.74%, corresponding to the first to fifth reuse. In addition, we also measure XRD and FTIR of material after recycling tests (Figure 6e-f). Surprisingly, all the chemical bonds in the g-CN nanosheets still remain after the fifth times reuse and the two diffraction peaks characterize the (100) and (002) planes of g-CN are also preserved.

Trapping tests (a), L-H Fit lines (b), recycling test (c), NO removal efficiency and NO2 conversion yield (d), FTIR spectra (e), and XRD patterns after recycling test of g-C3N4 nanosheets.
DISCUSSION
Figure 2a shows two typical peaks located at 13.2 and 27.4 corresponding to (100) and (002) planes which characterize for in-plane repeated tri-s-triazine units and triazine aromatic systems in g-CN structure 16. In Figure 2b, FTIR spectra showed that after undergoing a simple annealing route, melamine precursor formed more chemical bonds in the range from 1700 to 1200 cm that characterizes for stretching vibration of C-N heterocycles of g-CN nanosheets structure 17. In addition, the single peak centered at 810 cm in the FTIR spectrum of g-CN reveals the bending vibrations of the triazine units, which is one of the typical bonds of the g-CN matrix 17.
TEM images (Figure 3a-b) showed that the morphology of the g-CN is a 2D-structured material, including several singly exfoliated sheets. The construction of the 2D-structured nanosheets is due to the formation of strong C-N covalent bonds after undergoing an annealing route to form the nanosheets. Continuing the annealing treatment process, the bond between the nanosheets is the weak physical bond of van der Waals, so they can be easily exfoliated to create a single nanosheet, as shown in the results of the TEM images. Therefore, the morphology of g-CN is nanosheets with a large effective area that will be favorable for the photocatalytic process. From the results of modern analytical methods mentioned above (Figure 2, Figure 3), we can conclude that g-CN nanosheets material has been successfully synthesized by a simple thermal-exfoliation method.
Figure 4 presents the DRS profile of g-CN bulk and g-CN nanosheets. This result is perfectly reasonable as there will be a color change from the bright yellow of the bulk to the white color of the sheets. Moreover, the previous publications also showed that when the size of the material is reduced, it leads to a quantum confinement effect that causes the bandgap structure to increase 18, 19. Consequently, the enhancement in the redox ability of the material by increasing the bandgap and thereby facilitating the subsequent photocatalytic reactions.
In Figure 5a-b, the results of the photocatalytic NO removal efficiency of g-CN nanosheets are highest under solar irradiation at 70% humidity condition. This result can be explained as due to the high power of Osram, 300 W that can provide enough photon energy to activate the maximum photocatalytic reactions of g-CN nanosheets. In addition, g-CN nanosheets have a relatively narrow bandgap of about 2.7 eV 20. Consequently, g-CN nanosheets can harvest light in both visible and UV regions leading to the photocatalytic NO removal efficiency in the case Solar-OSRAM is the highest. Furthermore, from the results of Figure 5c-d, it can be confirmed that the humidity in the air directly influences the photocatalytic efficiency of the material. This phenomenon can be explained by the fact that in the photocatalytic process, the photogenerated hole (h) will oxidize the adsorbed HO molecules on the photocatalyst surface to form free radical OH that contributes to the photocatalytic reactions 5. Therefore, when the humidity is reduced, the number of HO molecules adsorbed on the photocatalyst surface is low, leading to a decrease in the amount of OH radicals. Consequently, the photocatalytic efficiency of the material is decreased drastically.
Figure 6a indicated that h plays an essential role in the photocatalytic process, and this result is completely consistent with the results of Figure 5c-d. On the other hand, e and O also play relative roles in photocatalytic reactions but not as dominant as h. In addition, we also calculate the reaction kinetic k value to quantiatively investigate the photocatalytic activity of g-CN nanosheets. The reaction kinetic k value is lowest in the case of KI. This means that by trapping h+, the photocatalytic reaction rate has been reduced significantly, or almost none happened. From the results of Figure 6a-b, we can solidly confirm that h plays a key role in the photocatalytic process. The results in Figure 6c-d can be explained due to the light-shielding effect that reduces the reaction area between the photocatalyst g-CN nanosheets and incident light during the photocatalytic process. After each recycling test, the NO gas is decomposed into NO ions, which will gather on the surface of the photocatalyst, causing the light-shielding effect that prohibits the interaction between the photocatalyst and the light, so decreases the photocatalytic efficiency5. From the results of Figure 6c-f, we can confirm that the g-CN nanosheets have very high durability and can be put into practical application for photocatalytic NO removal at a high concentration level with many times reuse.
From the analyzed results above, we propose a photocatalytic mechanism model for the 2D-structured g-CN nanosheets under solar light at 70% humidity by the following equations (Figure 7). When sunlight is irradiated with an incident wavelength greater than the energy of the bandgap, electrons (e) will move from the valence band (VB) to the conduction band (CB), leaving a hole (h) (
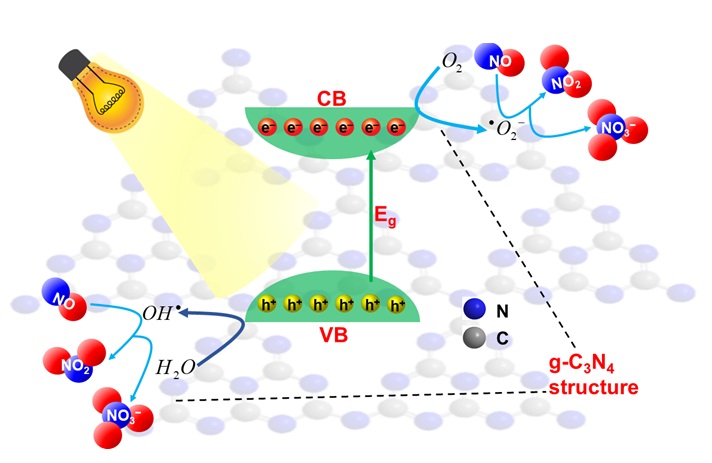
The proposed photocatalytic mechanism of g-C3N4 nanosheets for NO removal under solar irradiation.
CONCLUSIONS
In brief, we have successfully synthesized 2D-structured g-CN nanosheets by undergoing a simple thermal-exfoliation method. This is evidenced by the results of XRD, FTIR, TEM, and DRS results. The as-synthesized g-CN nanosheets achieve a very high photocatalytic NO removal at 48.27% under solar irradiation at 70% humidity thanks to the benefits of large specific surface area and narrow bandgap of the material. Moreover, the NO conversion yield is very low, only 9.44%, compared to 38.83% of the decomposition efficiency NO to NO ions. In addition, we also indicated that the h plays the most critical role in the photocatalytic reaction by trapping tests. Besides, the photocatalytic NO removal efficiency still reaches 45.03% after fifth times reuse. These results of XRD pattern and FTIR spectrum of photocatalyst after recycling tests still show all the characteristic diffraction peaks and typical chemical bonds in the g-CN nanosheets original state of the photocatalyst before recycling test. From the analysis results above, we can confirm that g-CN nanosheets possess a very high practical application potential for decomposing NO gas at high concentrations under solar irradiation.
Abbreviations
g-CN: graphitic carbon nitride
2D: two dimensions
XRD: X-ray diffraction
FTIR: Fourier transforms infrared spectroscopy
TEM: Transmission electron microscopes
DRS: UV-Vis diffuse reflectance spectroscopy
VB: valence band
CB:conduction band
h: hole
e: electron
ACKNOWLEDGEMENTS
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.02-2019.343.
Authors Contribution
Tran Hoang The Vinh: Investigation, Writing - Original draft preparation;
Huynh Cam Tu: Formal analysis;
Pham Van Viet: Writing — Review & Editing, Supervision, Funding acquisition.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.

