Gelation time characterization method for polyvinyl alcohol-based hydrogel
- Faculty of Chemical Technology, Ho Chi Minh City University of Food Industry (HUFI), 140 Le Trong Tan Street, Tan Phu District, Ho Chi Minh City, Viet Nam
- Department of Chemical Engineering, School of Biotechnology, International University, Ho Chi Minh City, Viet Nam
- Vietnam National University, Ho Chi Minh City, Viet Nam
Abstract
Introduction: Hydrogel and its gelation time have received great interest from material researchers and engineers. In this study, the method to evaluate the gelation time for hydrogel material was proposed.
Methods: The gelation test was conducted based on DV2T Brookfield viscometer and the newly designed gel timer (GT-2000). The method was applied to polyvinyl alcohol (PVA) solution with glutaraldehyde (GA) as a crosslinker. The blended hydrogel, including PVA and hydroxypropyl cellulose (HPC) was also prepared to confirm the versatility of the proposed method. The water uptake was conducted to confirm the relationship with the gelation time.
Results: With 3 wt% polymer solutions, both starting (torque is larger than 1%) and ending time (torque is larger than 90%) of the gelation could be detected. On the other hand, only the starting of gelation process could be measured with 1.5 wt% of the similar solution. When GA or the polymer concentration decreased, the gelation time could be reduced. By adding HPC to the PVA solution, the gelation time was reduced. The gelation time and water uptake were correlated with PVA hydrogel, while this type of relationship could not be detected in the PVA-HPC hydrogel samples. The addition of 1 wt% of HPC significantly improved the value of water uptake (more than 50 times).
Conclusions: In conclusion, the proposed gelation time method could be successfully applied to the PVA-based hydrogel and performed as a preliminary characterization technique for hydrogel materials.
INTRODUCTION
Hydrogel materials have received great interest from many researchers owing to their potential applications in agriculture1, medical, health care2, 3, or even in the cosmetic industry4. The ability to absorb a large quantity of water without being dissolved is one of the fascinating properties of hydrogel5. Hydrogel is typically synthesized based on highly hydrophilic polymers. The polymers could be originated from synthetic or natural sources6, 7. Polyvinyl alcohol (PVA) has been widely applied to fabricate hydrogel material because of its biocompatibility8, 9, 10, 11. In addition, several other types of polymers might be incorporated into the system to improve some properties of the hydrogel. The matrix must be crosslinked physically or chemically to form the 3D network12. The physical crosslink might be ionic interaction, crystallization force, or hydrogen bonding 13. Although the physical bond is considered to be weaker than the chemical crosslinking, the physically crosslinked hydrogel is a potential direction with many advantages. However, the newly developed approach via several freeze-thawing cycles might require special techniques and tools. The typical and more conventional method to create the covalent bonding between polymer chains is based on a chemical reaction with the presence of a crosslinker. For PVA and some polysaccharides, the hydroxyl functional groups were used to react with the aldehyde group of the crosslinker14, 15, 16. The starting materials are combined to form a mixture that is usually in a liquid state. The final product should be a solid or highly viscous mixture due to the crosslinking process. Therefore, the time required for handling the sample is very important for the hydrogel researchers or material engineers. It depends on several factors such as type, the concentration of crosslinker, or reactivity of the crosslinking reaction. To characterize the gelation time, the estimation could be made by simply tilting the solution to confirm whether it is still able to flow 17. The recent hydrogel researches for biomaterial still applied the vial inversion technique (i.e. the mixture is put in a vial and monitored by repeated inversion) 18, 19. The conventional method is able to be conducted with a small amount of sample. However, time for the gelation process could only be roughly obtained. The method strongly depends on the technician to judge whether it is the point of gelation. Moreover, if the time to start the gelation reaction is long enough, the error could be made because the gelation process might be very quick after the initialization. Therefore, more reliable method should be developed to find the suitable condition for the reaction.
Brookfield viscometer is common equipment in many material and chemistry laboratories. The main purpose is to obtain the viscosity of the liquid sample. The viscosity can be calculated from the spindle parameters and the torque moment value obtained during the rotationally stirring of a spindle in a solution. The increase of viscosity accompanies with the gelation because of the formation of 3D network. By monitoring the viscosity as a function of time, the gelation time can be determined. Brookfield DV-III viscometer has been applied to characterize the polyacrylamide-based hydrogel 20, 21. The mechanism includes using a suitable spindle connected to the viscometer. The spindle is dipped into the solution and stirred at a controlled speed. However, in these studies, instead of continuously recording, the viscosity values were obtained separately at different periods, which could cause some errors. Besides, with the traditional spindle design, when the gelation is completed, the spindle is stuck with both of test solution and viscometer. It is difficult and inconvenient to remove the spindles from the viscometer. Due to the hardening effect from the formed gel, the removal of the tested gel might damage the surface of the spindle. The cleaning process of the used spindle which is made of stainless steel and rather expensive, must be carefully conducted after each experiment. The company has just released a gel timer tool of GT-2000 which is designed for gelation time research. The tool is under a form of an additional part that can be used for the previously purchased viscometer. The spindle is made of the typical glass rod, which is easy to be cleaned and replaced.
In this study, the method to characterize the gelation time is proposed based on Brookfield rotary viscometer and gel timer tool. The PVA shall be the main hydrophilic polymeric matrix to synthesize the hydrogel. The physical crosslink PVA includes repeated freezing and thawing cycles which might be costly 8, 13, 22. The chemical crosslinking process is chosen to investigate the reliability of the developed method. The glutaraldehyde (GA) molecule is used to interconnect the polymer chains. The concentration of GA is varied to confirm the difference in gelation time. The ability to absorb water after crosslinking shall be conducted to reveal the relationship between the gelation time and the structure of the formed hydrogel. In addition, the cellulose’s derivative of hydroxypropyl cellulose (HPC) is added to the PVA solution to fabricate blended hydrogel. The gelation time measurement shall be applied to the PVA-HPC mixture. The blended PVA-HPC results shall be compared to the gelation time of the PVA hydrogel to confirm the versatility of the proposed methodology.
EXPERIMENTAL SECTION
Materials
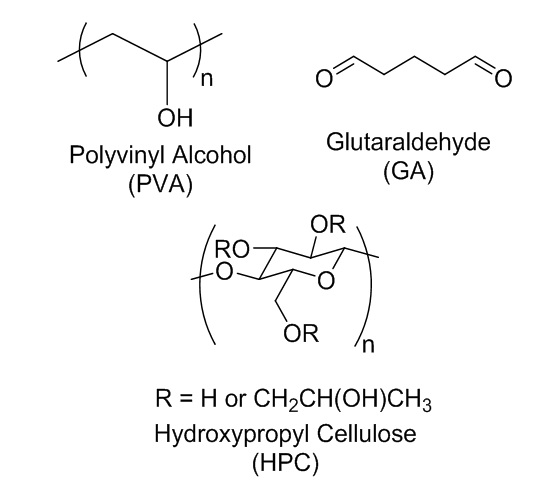
Chemical structure for PVA, HPC, and GA.
The chemical structures and abbreviations for the reagents used in this study were shown in Figure 1. PVA (85-90% degree of hydrolysis) and GA (50 wt% in water) were purchase from Shanghai Aladdin Bio-Chem Technology Co. LTD (China). HPC (viscosity 2%, 20 ˚C: 1000-5000 mPa.s) was purchase from FUJIFILM Wako Pure Chemical Corporation (Japan). Hydrochloric acid (HCl) was obtained from Duc Giang Chemicals Group (Vietnam). All chemicals were used as received.
Methods
Gelation time measurement was divided into two steps: polymer solution preparation and measuring time required for the solution to become a gel. The polymer powder was dissolved in 100 mL double distilled water under constant stirring for few hours to form a 3 or 1.5 wt% solution. GA solution was added to 50 mL of the polymer solution. Although the crosslinker was added and well dispersed in the matrix, the gelation was limited without acidic conditions 23. Then, 0.2 mL HCl (as a catalyst) was added, and the final mixture was stirred for 1 min before conducting the next experiment. The solution preparation was carried out at laboratory temperature.
The time until the gelation process occurred was obtained by using the rotational viscometer (Brookfield, model: DV2T, USA). The instrument was equipped with a gel timer (GT-2000, Brookfield) included a magnetic coupling and a glass rod. The picture of the viscometer installed with the gel timer is shown in Figure 2. In this measurement, the torque value was used instead of the viscosity, requiring several additional spindle parameters to be calculated. The spindle speed must be limited to detect the torque value at a higher viscosity because of higher resistance. With hydrogel, the viscosity might be very large. Therefore, the rotating speed was set at the minimum value (1 rpm). The multipoint sequence was set, and the torque data were recorded automatically every 5 s until torque reached 90%.

DV2T Brookfield viscometer and the GT-2000 gel timer.
The crosslinked hydrogel was immersed in distilled water for 24 h to dissolve the unreacted components. The crosslinked hydrogel was filtrated and dried in the oven (60 C) for 8 h. The dried gel (weigh is m) was immersed in 100 mL distilled water. The sample was periodically taken out of the water and put on the filter paper to wipe out the water on the surface. The sample was weighed again to receive the mass of the wet gel (m). The water uptake was calculated using the following equation:
The polymer solution with HCl and GA was prepared according to the same method used in the gelation time measurement procedure. 25 mL of the solution was poured into a petri dish (diameter 90 mm). The solvent was evaporated in ambient condition to form a hydrogel film. The samples for gel fraction measurement were prepared by cutting the hydrogel film into a round shape (diameter 2 cm). The samples were dried in an oven (60 ˚C) for 8 h and recorded the weight of m. The dried samples were immersed in 100 mL pure water for 24 h. Then, the mixture was filtered and dried in an oven (60 ˚C) for 8 h. The remained gel was weighed again to obtain m. The gel fraction was calculated using the following equation:
The gel fraction was repeated at least three times. ANOVA analysis (single factor, alpha: 0.05) was performed with MS Excel.
RESULTS
The concentration of PVA was fixed at 3 wt%, and the amount of GA was varied (2, 5, 10, and 15 μL). After stirring with 0.2 mL HCl for 1 min, the solutions were characterized with Brookfield rotary viscometer. With a standard spindle, the torque value detected by the viscometer was used to calculate viscosity. In this study, the gel timer (GT-2000, Figure 2) was used, and the torque value in % unit was reported. The torque value as the function of time was plotted, and the results were summarized in Figure 3.

Gelation time result of PVA solution (3 wt%) and varied amount of glutaraldehyde (2 (black square), 5 (red circle), 10 (blue triangle) and 15 (purple triangle) μL /50 mL polymer solution).
As can be observed in Figure 3, during the initial time, the torque value remained unchanged. When the gelation process occurred, the polymer solution could form the network structure, and the viscosity must be increased drastically. Accordingly, torque value after a duration of the stabilized state started to increase. The time for torque reached the value of 1% was reported in
Gelation time parameter resulted from measurement with PVA 3 wt% solution
|
Glutaraldehyde (μL) |
Starting time (1) (s) |
Ending time (2) (s) |
Gelling duration (3) (s) |
|
2 |
1215 ± 14 |
1467 ± 17 |
252 |
|
5 |
975 ± 31 |
1183 ± 12 |
208 |
|
10 |
785 ± 9 |
934 ± 11 |
149 |
|
15 |
735 ± 13 |
876 ± 10 |
141 |
The measurement was repeated in a similar approach except that the polymer solution concentration was reduced to 1.5 wt%. The result of gelation time was summarized in Figure 4. Similarly, the torque value of all samples started to increase after different periods of time, and it indicated that the gelling reaction begun to occur. However, torque could not reach 90% and sharply decreased after approaching a certain value.
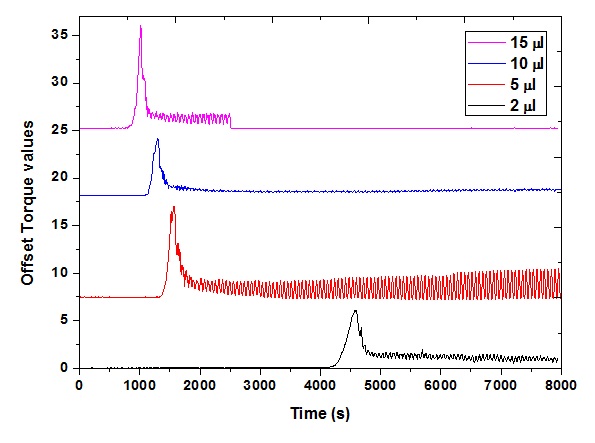
Gelation time result of PVA solution (1.5 wt%) and varied amount of glutaraldehyde (2 (black), 5 (red), 10 (blue) and 15 (purple) μL /50 mL polymer solution).
In the case of 1.5 wt% solutions, only the time for starting the process could be reported, and the results were summarized in
Gelation time parameter resulted from measurement with PVA 1.5 wt% solution
|
Glutaraldehyde (μL) |
Starting time (1) (s) |
The maximum torque (%) |
|
2 |
4320 ± 48 |
6.1 |
|
5 |
1410 ± 22 |
9.6 |
|
10 |
1170 ± 18 |
6 |
|
15 |
890 ± 27 |
10.8 |
To reveal the relationship of the gelation time measurement results and property of the final hydrogel product, the water uptake experiment, which can characterize the amount of water absorbed by the dried hydrogel, was conducted. The PVA hydrogel with more than 2 μL of crosslinker showed insignificant water absorption after 3 h sinking in distilled water. The water uptake results of two samples with 2 μL GA and the longest starting time for 1.5 and 3 wt% were shown in Figure 5.

Water uptake with PVA (black square-1.5 wt% and red circle-3 wt%) and glutaraldehyde (2 μL/50 mL polymer solution).
Using 1.5 wt% of PVA, water uptake value was improved (up to approximately 4 times) compared to sample prepared with 3 wt% solution. This result shows a good correlation to the obtained gelation time values. Indeed, the hydrogel samples of PVA 1.5 wt% and 3 wt% have starting times of 4320 and 1215 s, respectively. Furthermore, water uptake as the function of time also showed that water absorption with PVA 1.5 wt% samples could quickly be saturated in 40 min while the others could not reach the maximum water uptake within the characterized time.
The result is very promising as the gelation time measuring technique could be applied to characterize the PVA hydrogel. In the next experiment, the solutions formed by two kinds of polymer (PVA and HPC) were prepared and characterized gelation time to confirm the versatility of the proposed method.

Gelation time of PVA-HPC solution (3 wt%) with glutaraldehyde (2 μL/50 ml polymer solution); red circle: PVA(2 wt%)-HPC(1 wt%); black square: PVA(2.5 wt%)-HPC(0.5 wt%), blue triangle: PVA (3 wt%).
The 3 wt% polymer solution was prepared in a similar method to PVA cases except that HPC was added with PVA/HPC weight ratio of 2.5/0.5 or 2/1. The gelation time results of the mixture in comparison to PVA were shown in Figure 6, and the detailed parameters could be found in
Gelation time parameters for PVA-HPC solutions (3 wt%) with 2 μL of GA
|
PVA/HPC (Weight ratio) |
Starting time (1) (s) |
Ending time (2) (s) |
Gelling duration (3) (s) |
|
3/0 |
1215 ± 14 |
1467 ± 17 |
252 |
|
2.5/0.5 |
720 ± 24 |
949 ± 13 |
229 |
|
2/1 |
475 ± 22 |
688 ± 11 |
213 |
The water uptake experiment of the hydrogel formed by the mixture of PVA and HPC was also conducted and the percentage of water absorbed as the function of time as shown in Figure 7. Although the addition of 0.5 wt% HPC could cause a noticeable decrease in starting gelation time, there is no sign of improvement in the water uptake results. On the other hand, with 1 wt% of HPC, the formed hydrogel showed a significant increase in water uptake (up to 50 times). In addition, the gel was quickly saturated with water within 10 min.

Water uptake of PVA/HPC hydrogel (3 wt% polymer solution); blue triangle: PVA(2 wt%)-HPC(1 wt%); red circle: PVA(2.5 wt%)-HPC(0.5 wt%), black square: PVA (3 wt%).
The gel fraction results of blended PVA-HPC hydrogel samples are shown in Figure 8. In general, there is a small decrease in gel fraction values when the solution concentration was reduced to 1.5 wt%. Besides, the value is also slightly decreased as more HPC was added into the system.

Gel fraction of PVA-HPC hydrogel with difference ratios (PVA(2 wt%)-HPC(1 wt%) and PVA(2.5 wt%)-HPC(0.5 wt%)) prepared by 3 or 1.5 wt% polymer solution(Glutaraldehye concentration is 2 μL/50 mL polymer solution (P < 0.001).
DISCUSSION
As can be seen, the proposed method could be applied to obtain the gelation time quantitatively, which is better and contains fewer human errors than the estimation techniques in the previous articles 17, 18, 19. The gel timer tool (GT-2000) can be easily detached from the viscometer by magnetic coupling. For a material with a given viscosity, the resistance to rotate a spindle depends on its size and shape. If the contacting area of the used spindle is larger, the resistance is larger. As a result, the limitation of viscosity which still can be detected by the viscometer, is smaller. The glass rod spindle in this study is an advantage to detect a highly viscous sample during the gelation. In addition, the glass rod contacted directly to the mixture could be easily washed or replaced at a low cost. In the other reports 20, 21, the viscosity was plotted as a function of time to determine the gelation time. Viscosity is calculated according to the below equation
where η is viscosity. τ and γ are shear stress and shear rate, respectively.
However, a polymer solution is non-Newtonian fluid even at a dilute concentration and viscosity24. The viscosity depends on the shear rate (γ), which is a function of the angular velocity of the used spindle. Therefore, the viscosity of a polymer solution is varied with the preset spindle speeds. The variety is defined by polymer rheology. In addition, during the gelation process, the molecular weight is increased, which might change the rheological characteristic of the materials. On the other hand, the gelation time technique requires the consistency in measuring conditions (type of spindle and its speed). It is difficult and impractical to change the speed of the spindle during the gelation process. As a result, the viscosity during the formation of gel could not be precisely calculated. The shear stress in the equation is related to the torque value, which represents the needed force to maintain the same rotating speed. It is a practical value that can be directly obtained by the viscometer without any assumption. The time dependence of the viscosity can be evaluated through the characterization of the sample’s torque. Besides, the main purpose of the developed technique in this study is to receive the gelation time. Therefore, instead of viscosity, torque is more suitable to investigate the crosslinking process of hydrogel material. Another advantage in this method compared to the previous reports 20, 21 is the ability to continuously detect the torque signal. The data interval of the torque-time graph could be easily controlled by changing the viscometer setting.
When the gelation process is initiated, viscosity and torque are quickly increased. The maximum torque moment that can be detected by the viscometer is limited by both equipment’s capacity and spindle design. In general, a larger contacting surface of the used spindle cause higher torque with the same sample’s viscosity and rotating speed. The rod-like shape of the GT-2000 has smaller contacting area, and the gelation process might be observed during a longer time than the traditional spindle. This is an advantage of the proposed method for hydrogel characterization. However, when the torque value is exceeded 90%, the gelling is reaching the maximum capacity of the equipment. In addition to the errors which might be caused by the tools, this region (90-100%) provides less information about the sample’s gelation. Therefore, the concept of “ending time” used in this study might be misunderstood as the gelling reaction still continued to occur in the sample even after the measurement. Indeed, the solidification process of the hydrogel sample continuously proceeded in the next 24 h at room temperature. However, the duration from the beginning of the reaction to the point of the highest torque value could still provide valuable information about the reaction speed. As can be observed, the gelling duration time is shortened as the concentration of the crosslinking reagent increased. The result indicated that the speed of forming the network structure was faster.
The torque value is directly dependent on the density of crosslinking in the hydrogel matrix. However, unlike the physical crosslinking, which might be unbonded, the irreversible process, in this case, is based on chemical bonding reaction. Firstly, the decrease of the torque value after reaching a certain value for 1.5 wt% solutions (Figure 4) was assumed to be an indication for breaking of the hydrogel samples when the crosslinking had been completed. However, all samples continued to solidify by storing at room temperature for one or two days. Therefore, the reaction might not be accomplished during the measurement time. As a result, there are two possible reasons for the obtained phenomenon in the 1.5 wt% samples. The mechanical breaking at the contacting area between the glass rod of the gel timer (Figure 2) and the under-formed hydrogel is the first reason causing the reduction of torque. Because of using the lower concentration of polymer, compared to the sample with 3 wt%, the network was relatively loose before the gelation was accomplished. Followed the mechanical breaking caused by the glass rod, the second reason evolved. The amount of crosslinker was maintained (at 2 μL) while the polymer concentration was reduced. The crosslinking density was increased accordingly when all of the crosslinker molecules had attended into the reaction. When the reaction continuously occurred, the hydrogel gradually shrank with a higher ratio (due to the low concentration). As a result, the contacting area between the rotating rod of the viscometer and the sample was gradually reduced. By the time of sufficient crosslinking bond, the rod could not detect the increase of viscosity in the sample, and a sharp decrease of torque might appear.
The method of gelation time measurement by utilizing Brookfield rotary viscometer has shown many advantages, including simple technique, low cost, and being able to predict a deeper understanding of the crosslinking process. The time to start the gelling reaction provides useful information to handle the solution before it becomes a gel. In addition, it might be an indicator of the crosslinking density as well as the network structure inside the final hydrogel. With the same type of polymer, the formed hydrogel with a longer starting time might accompany a loosely crosslinked matrix. This prediction is supported by the water uptake (Figure 5) and the starting time (
To explain the reduction of gelation time by adding HPC to PVA system, the chemical structures of the compounds have to be taken into account. Each aldehyde group of GA molecule will react with two hydroxyl groups of the polymer to form the network structure16. Therefore, the gelation time is strongly dependent on the number of the -OH groups. HPC with chemical structure possesses a large concentration of -OH on the polysaccharide chain (Figure 1) might provide more suitable conditions for the crosslinking reaction. The gelling duration time for the PVA-HPC mixture also reported. Interestingly, although the time required to begin the reaction was shortened by approximately 2.5 times by only adding 1 wt% of HPC, the gelling duration was reduced slightly. In the case of varying the GA concentration (
There is a slight reduction in the water uptake after saturation (Figure 7). This is due to the loss of the hydrogel sample during the measurement because the gel has to be taken out periodically for weighing. Although the gel could not be dissolved due to the 3D network, it is possible that the gel might be broken apart. This suggestion is supported by the gel fraction results (Figure 8). As can be observed, the gel fraction of all of the samples was higher than 70%. It indicated that the hydrogel was successfully formed even when the polymer solution was diluted to 1.5 wt%. Therefore, the slight decrease of water uptake is due to the weak mechanical property, and this is another evidence for the loose network of the PVA/HPC (2/1) hydrogel.
CONCLUSIONS
The method of measuring gelation time has been developed and applied successfully to quantitatively characterize the crosslinking process of the PVA-based hydrogel. The method utilized Brookfield rotary viscometer with gel timer (GT-2000) showed its advantages, including low cost, versatility, and more convenience in cleaning/detaching protocol than the previous techniques. The proposed method was able to detect the gelation process continuously via torque value rather than the unprecise viscosity. By applying the newly developed technique, the times to start the crosslinking of the PVA solution obtained with both of 3 and 1.5 wt% solutions were found to be shortened by using less amount of GA. However, the value of torque larger than 90% could not be detected with 1.5 wt% samples. The addition of HPC causes a significant reduction in the gelation time. By adding 1 wt% of HPC to PVA solution, the amount of absorbed water in the formed hydrogel was significantly increased (50 times). The starting gelation time obtained by the proposed method could only be correlated well with water uptake results when the single polymeric component of PVA was used.
ACKNOWLEDGEMENTS
This work was funded by Ho Chi Minh City University of Food Industry (contract number 40 HĐ/DCT, date: Sep 9 2020).
LIST OF ABBREVIATION
PVA: Polyvinyl Alcohol
GA: Glutaraldehyde
HPC: Hydroxypropyl Cellulose
COMPETING INTERESTS
The author(s) declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Giang Ngoc Ha developed ideas of gelation time measurement. Giang Ngoc Ha and Truong Thi Phuong Dung wrote the manuscript. The other authors contributed equally in conducting experiments, analyzing and discussion the obtained results.
All authors have read and approved the final version.

