Improving the electrochemical performance of hard carbon||NaxNi1=3Mn1=3Co1=3O2 full-cell by presodiation methods
- Applied Physical Chemistry Laboratory (APCLAB), Faculty of Chemistry, University of Science, Ho Chi Minh City
- Viet Nam National University Ho Chi Minh City (VNU-HCM), Ho Chi Minh City, Viet Nam.
- Viet Nam National University Ho Chi Minh City (VNU-HCM), Ho Chi Minh City, Viet Nam
- Faculty of Chemistry, University of Science, Ho Chi Minh City
Abstract
Introduction: We study the electrochemical behaviors of sol-gel NaxNi1=3Mn1=3Co1=3O2 (NaNMC) material assembled in sodium half-cell and full-cell configuration, focusing on the cycling performance.
Method: The crystalline structure and the lattice parameters of synthesized NaNMC were characterized by using X-ray diffraction (XRD). The electrochemical characterization of NaNMC sample was investigated in sodium-ion half-cell by cyclic voltammogram (CV) and galvanostatic cycling with potential limitation (GCPL). To improve the reversible capacity of sodium-ion full-cell, we suggested two presodiation methods to reduce the irreversible capacity of the hard carbon before assembling full-cell: i) the chemical method and ii) the electrochemical method.
Results: In the half-cell, the specific capacity of NaNMC is about 106 mAh.g-1 and well maintained 88 mAh.g-1 after 100 cycles in a potential range of 2.0 – 4.0 V. When assembling the full-cell with a pristine hard-carbon, the cell capacity of only 30 mAh.g-1 but quickly decreased after several cycles due to large irreversible capacity of hard carbon in the initial cycle. As a result, both methods significantly increased ICE (Initial Coulombic Efficiency) of hard carbon by over 90% and enhanced the capacity of the full-cell. In particular, the reversible capacities of full-cell based on presodiated hard carbon (HC) were twice higher than those with pristine HC (75 mAh.g-1 and 65 mAh.g-1, respectively, for the electrochemical method and the chemical method).
Conclusion: These encouraging results indicate an accessible solution to overcome the low ICEs of hard carbons, leading to improving the sodium-ion full-cell's reversible capacity.
INTRODUCTION
Electrochemical energy storage has been demonstrated as versatile technology because of its high energy conversion efficiency, relatively compact size, and fast response. Furthermore, by integrating with the battery technologies, the energy generated can be stored and conserved to be widely deployed in multiple applications. Among various electrochemical energy storage systems (EESSs), lithium-ion batteries (LIB) dominate all mobile electronic devices and electric vehicles. However, the limited lithium resource and the high cost of LIB were future challenges, especially when large-scale renewable energy storage became popular1. Therefore, sodium-ion battery (SIB) has been prospectively considered the next generation beyond LIB technology due to the similarity between lithium and sodium chemistry, the redox mechanism, the cost-effectiveness, and the earth's abundance of sodium resources (more than 1000 times higher than that of lithium)2, 3.
Regarding typical cathode materials, NaNiMnCoO (NaNMC) layered oxide is a promising candidate for sodium-ion batteries owing to an isotope structure of α-NaFeO and a counterpart of LiNiMnCoO as well4, 5, 6. This tertiary oxide has the main redox couple of Ni/Ni contributing to the overall capacity, while the presence of Co helps to enlarge the working voltage of NaNMC up to 4.0 V and Mn maintains the structural stability of NaNMC. NaNMC was first synthesized via the sol-gel method followed by calcinating at 900 C and showed O3 purity phase that delivered a reversible specific capacity of 120 mAh.g at a current density of C/10, in a potential range of 2.0 – 3.75 V7. The material O3-NaNMC was also obtained by the hydroxide co-precipitation method that exhibited a capacity of 141.1 mAh.g at a current density of 15 mA.g in a wider voltage range of 1.5 – 4.1 V8. In general, the material NaNMC both single-phase O3 or mixture phases, owned superior cycling stability that very appreciated for using as cathode material for SIBs 9, 10.
Recently, hard carbon (HC), a nongraphitic carbon, has been reported as an ideal anode material for SIBs11, 12, 13. However, HC serving as an anode material possesses several defects, large hysteresis during Na ion insertion/disinsertion, low initial Coulombic efficiency (ICE, < 80 %), poor electronic conductivity originating from its disordered texture. The low ICE results from large irreversible capacity in the first cycle, which does not attractive for practical utilization. This unexpected capacity is mainly derived from Na ions trapping in the closed micropores in the HC matrix and solid electrolyte interphase (SEI) layer formation 14, 15, and thus leading to the increase of surface area as well as the reduction of volumetric capacity. Many attempts to resolve the low initial Coulombic efficiency (ICE) of carbonaceous anode material have been proposed for lithium-ion batteries, such as i) prelithiating the anode by a self-discharge mechanism16, ii) prelithiating by electrical shorting with outer circuit equipment17 and iii) prelithiating by electrolytic methods17, 18. Prelithiation (or presodiation) is the most practicable technique to compensate for the initial irreversible capacity. However, there are still some potential concerns about the time, cost, and universality when adapting for hard carbon anode in SIB.
In this study, the electrochemical properties of NaNMC material were firstly reported in half-cell and full-cell configuration with a hard carbon anode. Then, the performance of full-cell hard carbon||NaNiMnCoOwas continuously improved by increasing the Coulombic efficiency of the hard carbon-based anode of full-cell by two presodiation methods such as the chemical method 19 and the electrochemical method20.
EXPERIMENTAL
Preparation of cathode material
Layered NaNiMnCoO (NaNMC) with a nominal stoichiometry was prepared by the sol-gel method. Firstly, precursor solution was prepared by mixing NaOH (Merck, > 99%), Ni(NO).6HO (Sigma-Aldrich, > 99%), Co(NO).6HO (Sigma-Aldrich, > 99%), and Mn(OAc).4HO (Acros, > 99%) and citric acid as a chelating agent in the molar ratio 1.05:0.33:0.33:0.33:1.00, respectively and adjust pH by adding solution NH 25% (Kanto Chemical) then heated at 80 C until a transparent viscous gel forms. Then, the gel was decomposed at 400 C for 12 hours then calcined at 900 C for 12 hours under an air atmosphere with intermediate grounding. The crucible was removed from the furnace intermediately after calcinating and directly transferred into an inert atmosphere glovebox to the ground and stored to avoid any reaction with moisture and carbon dioxide.
Material characterization
The crystalline structure of NaNMC materials was determined by X-ray diffraction performed on D8 Advanced (Bruker). The materials were radiated by CuKa source (λ = 1.5814 Å) in a 2θ angle range of 10 - 70 within 0.02 /step/0.25 second rate. XRD patterns were analyzed by X’Pert Highscore Plus software (version 3.0).
Electrode preparation
Cathode preparation was conducted in an argon-filled glovebox (GP-Campus, Jacomex). The electrode composite includes active material, acetylene black carbon C65, and binder of poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) in a weight ratio of 80:15:5 was mixed in N-methyl-2-pyrrolidone (NMP) solvent to form slurry, which was then coated on an Al foil. The coated foil was dried at 110 C on a hot plate for 12 hours in the glovebox before being punched into 12 mm diameter discs. Mass loading of the electrode is about 2-3 mg.cm and 4-5 mg.cm when used in half-cell and full-cell, respectively.
Negative electrode hard carbon was prepared by the same process of making cathode electrode consist of hard carbon, acetylene black carbon C65, and PVDF at a weight ratio of 90:5:5 was mixed in N-methyl-2-pyrrolidone (NMP) solvent to form a slurry which was then coated on an Al foil. Mass loading of the electrode is 2-3 mg.cm.
Presodiation of the hard carbon electrode
The chemical method
In this way, the spontaneous sodiation would be initiated by a short circuit constructed between HC electrode and Na metal foil in the presence of electrolyte19. Indeed, presodiated hard carbon electrode occurred inside a Swagelok cell (Figure 1) wherein Whatman fiber was placed on one terminal, then an anode HC film was placed on the side upwards to avoid deconstruction of the electrode. A sodium slice was put on the bottom of the cell before screwing the final terminal. The contacting time was at least 30 minutes to get high-efficiency sodium intercalated in the HC structure19. After carefully removing Na metal foil, the presodiated HC electrode was finally coupled with NaNMC cathode making up a full cell.

Swagelok type cell for presodiated hard carbon by the chemical method.
The electrochemical method
The hard carbon electrode was initially cycled in sodium-ion half-cell at least three initial cycles at 0.1 C rate to activate the intercalated sites of HC structure, then terminated at 0.1 V on discharging. After that, the half-cell was disassembled to remove the HC electrode for making a full-cell with NaNMC cathode. The main purpose of using “cycled” HC is to eliminate maximum its irreversible capacity in some first cycles. Such a treatment process of hard carbon is conventionally designated as the presodiation20.
Cell assembly
Coin-cell CR2032 was assembled in an argon-filled glovebox using a prepared electrode, a sodium disk as a counter electrode and reference, two Whatman layers as a separator, and the electrolyte is the solution of 1 M NaClO in propylene carbonate (PC) with 2% fluoroethylene carbonate (FEC) additive. The hard carbon was considered as an anode in full-cell configuration. Electrochemical testing was controlled by multi-channel MPG-2 (Biologic, France). Cyclic voltammetry (CV) was conducted at the scan rate of 100 µV.s in a potential range of 2.0 – 4.0 V. Charge-discharge performance was processed in galvanostatic mode at 0.1 C (1 C corresponding to a current value of 240 mA.g).
RESULTS
Structure determination of NaNMC

X-ray diffraction pattern of synthesized NaNMC.
In Figure 2, the X-ray diffraction pattern of synthesized NaNMC samples exhibit peaks belonging to P2-phase with space group P6/mmc (PDF No: 00-054-0894). Additionally, there are still involves some additional peaks confirming the existence of the second phase that asserted belong to P3-phase with space group R3m (PDF No: 00-054-0839). Besides the signals of expected phases, we also observed the unexpected peaks marked with ‘*’ of impurity NiO (the peak position at 2θ = 37, 43 and 63). As known, the formation of NiO is unavoidable during the synthesis of NaNMC9. Based on the peak intensity, the synthesized sample contains mainly the active component of P2- and P3-phases with estimated percentages are of 56 % and 44 %, respectively. The lattice parameters calculation for each phase is shown in
The lattice parameters of synthesized NaNMC.
|
Phase ID |
Space group |
Lattice parameters |
Intensity ratio (%) |
|
P3 |
R3m |
a = b = 2.8455 Å c = 16.6.019 Å V = 116.41 Å3 |
44 |
|
P2 |
P63/mmc |
a = b = 2.8401 Å c = 11.0126 Å V = 76.93 Å3 |
56 |
Electrochemical characterization of NaNMC
The electrochemical characterization of the NaNMC sample was investigated in sodium-ion half-cell. In Figure 3, the voltammogram curve in a potential range of 2.0 – 4.0 V presents four couple peaks located at 2.97/2.40 V, 3.19/3.11 V, 3.45/3.38 V, and 3.74/3.63 V. The narrow between oxidation/reduction peak couples and high intensity revealed a reversible Na-intercalation/deintercalation process. Interestingly, the CV curve of the P3/P2-NaNMC in this work is with O3-NaNMC of Hwang’s report 8, indicating the similarity in the phases' electrochemical properties.
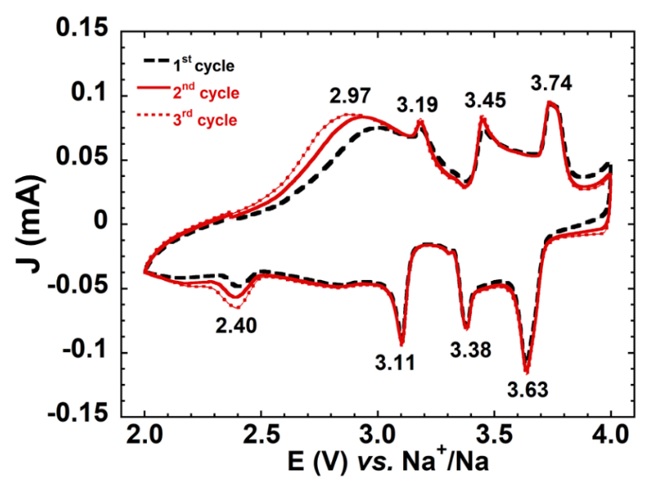
Cyclic voltammogram of NaNMC cathode in the voltage range of 2.0 – 4.0 V at the scanning rate of 100 μV/s.
Moreover, the voltage composition curve for NaNMC both in the discharge and charge stage revealed a stair-like profile indicating a reversible Na-driven structural transition (Figure 4a). The initial discharge capacity of the double-phase was 106 mAh.g in a voltage range of 2.0 – 4.0 V. After 100 cycles; the discharge capacity decreased to 88 mAh.g with 82.2 % retention of the initial capacity (Figure 4b). Additionally, the Coulombic efficiency remains approximative 100% during the cycle. The voltage profile maintained its feature over 100 cycles, as shown in Figure 4b, as result of the stability of the structure of component phases.
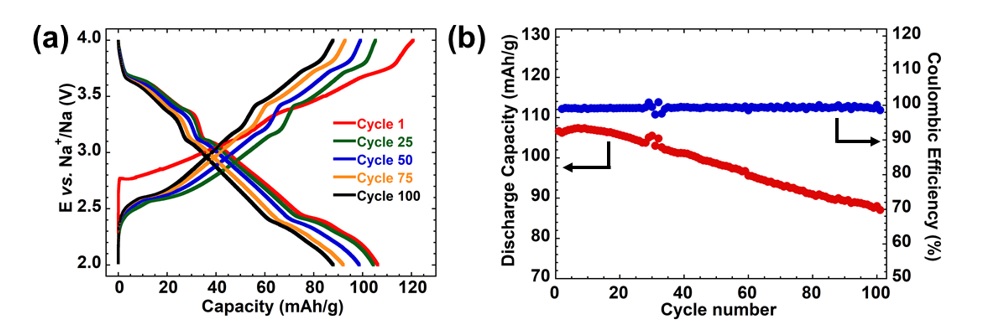
Charge/discharge curves of NaNMC electrode using 1M NaClO4 in PC + 2 %vol. FEC at 0.1 C (a); Capacity retention and Coulombic efficiency of half-cell NaNMC Na (b).
Full-cell performance based on presodiated Hard Carbon
Previously reported, NaNMC electrode could be an effective cathode for developing practical sodium-ion batteries8. To understand the practical realization of NaNMC electrode, Na-ion full-cell is assembled using NaNMC cathode and hard carbon as an anode. To make a SIB full-cell matching capacity between cathode and anode is an essential step. The good full-cell shouldn’t have any large irreversible capacity related to the loss of sodium ions for solid electrolyte interphase (SEI) or the side reaction between the electrode and electrolyte. In this study, we optimize the ratio between cathode and anode material to achieve full capacity in a sodium-ion cell. However, the hard carbon anode in our work delivers an initial discharge capacity of 240 mAh/g (Figure 5a) in NaClO 1 M/ PC + 2% FEC electrolyte. Therefore, it displays a very high initial irreversible capacity of about 65 mAh/g. To overcome irreversibility, we suggest two following approaches: (1) improving the initial Coulombic efficiency of the hard carbon-based anode by the chemical method (directly contact with sodium metal)21; and (2) presodiated hard carbon by the electrochemical method20.

The first, second, and fifth charge/discharge curve of pristine hard carbon (a) and 30 mins pre-sodiated hard carbon (b) in sodium half-cell. The charge/discharge curve of pre-sodiated hard carbon NaNMC (c) full-cell and corresponding cycle performance of the two types of full-cell at 0.1 C (d).
In the chemical method, galvanostatic charge-discharge tested at C/10 rate was performed in Na half-cell using the pristine (Figure 5a) compared to the cell using 30 minutes presodiated (Figure 5b) hard carbon electrode. During the first Na intercalation, the pristine shows two short and flattened slopes and relatively long plateaus, indicating three different intercalation processes. However, the presodiated sample exhibits only one slope and a flat region around 0.1 V. Moreover, the initial discharge capacity of pristine and presodiated hard carbon was 250 mAh.g and 150 mAh.g, respectively, with an initial Coulombic efficiency (ICE) of 73 % and 90 %. Thus, presodiation process improves the ICE hard carbon by compensating the irreversible capacity loss related to SEI layers formation in the initial cycle. Figure 5c shows the three first charge-discharge cycles of presodiated hard carbon || NaNMC full-cell. The Coulombic efficiency is approximately 80% with a specific capacity of 65 mAh.g was obtained in a potential range of 0.50 – 3.95 V at a current of C/10 based on the mass of active cathode material. Simultaneously, the cycle performance is also improved, as shown in Figure 5d. The presodiated hard carbon displayed an initial discharge capacity of 65 mAh.g and decreased to 15 mAh.g after 50 cycles (which means an average capacity loss of 1mAh/g per cycle). So, these results reconfirm that the presodiation process did not help improve the inherent electrochemical performance apart from reducing the irreversible Na consumption occurring in the initial cycle.
In the electrochemical method, a hard carbon electrode exhibits a reversible capacity of 175 mAh.g and an initial Coulombic efficiency of about 73 % (Figure 6a). In addition, the first three charge/discharge curves of half-cell are well-overlapped, indicating an excellent reversible sodium-ion insertion/extraction behavior. The full-cell using pristine hard carbon NaNMC shows the initial Coulombic efficiency of 40 % with a specific capacity of 26 mAh.g based on the mass of the cathode. In contrast, the presodiated hard carbon NaNMC full-cell exhibited a Coulombic efficiency is approximately 80 % with a specific capacity of 75 mAh.g based on the mass of active material of the cathode. Thus, the presodiated hard carbon cell helps to improve the discharge capacity of the full-cell. Simultaneously, the cycle performance is also beneficial from this treatment of hard carbon, as shown in Figure 6d. Indeed, the capacity retention enhanced from 38 % (for 30 cycles) in the case of the pristine anode to more than 50 % (for 50 cycles) when using pre-sodiated one.

Three first charge/discharge curves of Na pristine hard carbon half-cell (a). Charge/discharge curves of the pristine hard carbon NaNMC full-cell (b) and charge/discharge curves of presodiated hard carbon NaNMC full-cell (c). Corresponding cycle performance of the two types of full-cell at 0.1 C (d).
DISCUSSION
CV curves were shown in Figure 3 that the peak is associated with the typical reversible electrochemical intercalation/de-intercalation process of Na into the layered structure. The couple peaks located at 2.97/2.40 V Na/Na displayed the reversible redox process of Mn/Mn22. In the between 3.0–4.0 V region, the peak pairs at 3.19/3.11 V and 3.45/3.38 V Na/Na showed the contribution of Ni/Ni and Ni/Ni redox pairs, respectively 21. A high voltage redox couple at 3.74/3.63 V Na/Na corresponds to the Co/Co redox reaction.
In the chemical method, it is well known that spontaneous sodiation could be performed a short circuit is constructed between a hard carbon electrode and sodium metal foil in the presence of the electrolyte 19. When the sodium metal touches hard carbon without external pressure, a short circuit appears on the hard carbon launching for electrochemically presodiated. In this system, the electrolyte acts as sodium ion reservoir and materials to be electrochemical reduced to form SEI layer. Therefore, the solid electrolyte interphase (SEI) layer could be initially formed on the hard carbon surface as similar as the cycling process in regular batteries. The method is interesting that the SEI layer formed without redox reaction and consumption of sodium ions extracted from the cathode. Thus, the sodium ions extracted from the cathode are available to insert into hard carbon, improving the full-cell's specific capacity. In practice, the method has improved the Coulombic efficiency of hard carbon anode and the specific capacity of the full-cell, but the specific capacity still decreases significantly as probably the sodium ions detached from the sodiated hard carbon.
The hard carbon anode was initially cycled in half-cell with sodium metal as the counter for three cycles in the electrochemical method. Subsequently, the half-cells are disassembled to remove the presodiated hard carbon and reload it in fresh full cells with NaNMC cathode. Indeed, using “cycled” electrodes to make full-cell aims to eliminate the irreversible capacity loss in hard carbon electrodes during some first charge-discharge cycles. Due to the early SEI layer formation, the initial Coulombic efficiency of the hard carbon and the full-cell were improved. The full-cell capacity was also improved in the same way as mentioned before. It is noticed that the full-cell using electrochemical presodiated hard carbon anode exhibits more stable cycling than the cell using chemical presodiated and the pristine hard carbon, revealed that the sodium ions were held more tightly inside the microstructure of hard carbon and pretreatment of hard carbon using a sodiation cycling process electrode is conventionally designated as the pre-sodiation20.
CONCLUSIONS
NaNiMnCoO(NaNMC) was successfully synthesized by a sol-gel process followed by a calcination step at 900 C for 12 hours. Galvanostatic profile of NaNMC at C/10 rate exhibited a specific initial capacity of 106 mAh.g and the value of 88 mAh.g after the 100 cycle in a potential range of 2.0 – 4.0 V. However, the capacity of hard carbon || NaNMC full-cell showed only 30 mAh.g and quickly decreased after several cycles due to large irreversible capacity of hard carbon during the first charging-discharging process. To improve the initial Coulombic efficiency of hard carbon anode for the full-cell with NaNMC, two approaches such as chemical and electrochemical methods, were reported. Both methods significantly increased the initial Coulombic efficiency of hard carbon, such as 90 % (chemical method) and 94 % (electrochemical method). In the chemical method, the full-cell can exhibit a reversible capacity of 65 mAh.g. Regarding the electrochemical method, the full-cell show a reversible capacity of 75 mAh.g. These encouraging results indicate an accessible solution to overcome the low ICEs of hard carbons, leading to improved the sodium-ion full-cell's reversible capacity.
LIST OF ABBREVIATIONS
CV: Cyclic Voltammetry
EESs: Electrochemical Energy Storage Systems
FEC: Fluoroethylene Carbonate
HC: Hard Carbon
ICE: Intial Coulombic Efficiency
LIB: Lithium-ion battery
NaNMC: NaNiMnCoO
NMP: N-methylpyrrolidone
PC: Propylene carbonate
PVDF-HFP: Poly(vinyliden fluoride)-co-hexafluoropropylen
SEI: Solid Electrolyte Interface
SIB: Sodium-ion battery
XRD: X-Ray Diffraction
COMPETING INTERESTS
The authors declare that they have no competing interests.
Author contributions
Minh Le Nguyen: suggesting the idea and methods, conducting experiment and preparing manuscript.
Hoang Van Nguyen: suggested the idea and method, edit the manuscript and submit the manuscript.
Man Van Tran, Phung My Loan Le: lecture contribution, complete the manuscript.
Acknowlegdement
This research is funded by Vietnam National University, Ho Chi Minh City (VNU-HCM) under grant number C2020-18-10.

