Further report on the chemical constituents of the n-hexane extract of Leonotis nepetaefolia (L.) R. Br (Lamiaceae)
- Department of Organic Chemistry, University of Science, Vietnam National UniversityHo Chi Minh City, Viet Nam
Abstract
Introduction: Leonotis nepetifolia (L.) R. Br, a species of the family Lamiaceae, has some interesting biological activities, such as antibacterial, antioxidant, and anti-inflammatory activities. This paper describes the structural elucidation of seven compounds isolated from Leonotis nepetifolia collected in Xuyen Moc district, Ba Ria–Vung Tau Province, in June 2018.
Methods: Phytochemical investigations of the n-hexane extract of Leonotis nepetifolia led to the isolation of seven pure compounds. Their chemical structures were elucidated by extensive MS, 1D and 2D-NMR spectroscopic analysis and comparison with previously published data.
Results: Seven compounds, namely, (E)-10-oxooctadeca-8-enoic acid (1), ergosterol peroxide (2), turmeronol A (3), methyl (E)-3-(3,4-dimethoxyphenyl)propenoate (4), methyl 4-hydroxybenzoate (5), methyl (E)-3-(4-hydroxy-3-methoxyphenyl)propenoate (6), and alpinumisoflavone (7), were identified.
Conclusions: Although these compounds are known in other species, this is the first time they have been reported in Leonotis nepetifolia.
INTRODUCTION
(L.) R. Br (family Lamiaceae) is native to tropical Africa and southern India. In traditional medicine, is used to treat bronchial asthma, diarrhea, fever, rheumatism, and malaria as an analgesic in menstrual cramps1. Previous studies of this plant reported that extracts and compounds isolated from this species possessed interesting biological activities: antioxidant2, inhibition of MCF-7 (human breast cancer cell line) and Hep-2 (human larynx epithelioma cancer cell line)3, anti-inflammatory4, and antibacterial5 activities. Previous phytochemical studies of L. nepetifolia showed the presence of laballenic acid, allenic acid, labdane diterpenoids, iridoids, and coumarins6, 7, 8, 9.
In the search for chemical constituents of , herein, we reported a continuation study on an -hexane extract to isolate seven compounds: ()-10-oxooctadeca-8-enoic acid (1), ergosterol peroxide (2), turmeronol A (3), methyl ()-3-(3,4-dimethoxyphenyl)propenoate (4), methyl 4-hydroxybenzoate (5), methyl ()-3-(4-hydroxy-3-methoxyphenyl)propenoate (6), and alpinumisoflavone (7). Their chemical structures were elucidated by extensive MS, 1D and 2D-NMR spectroscopic analysis and comparison with previously published data.
MATERIALS AND Methods
General experimental procedures
HR–ESI–MS was recorded on an HR–ESI–MS MicrOTOF–Q mass spectrometer. The LC–MSD was recorded on an 1100 Series LC/MSD Trap SL mass spectrometer. The LC–MS/MS was recorded on a TSQ Quantum Access MAX triple quadrupole mass spectrometer. Optical rotations were measured on a Krüss (Germany) polarimeter with a tube length of 0.5 decimetres.
The H-NMR 500 (MHz), C-NMR (125 MHz) and 2D-NMR spectra were recorded on a Bruker Avance 500 spectrometer. Chemical shifts are expressed in ppm using a residual solvent signal as the internal reference (CDCl d 7.26, d 77.1).
Thin-layer chromatography (TLC) was carried out on precoated silica gel 60 F or silica gel 60 RP–18 F (Merck), and the isolated compounds were visualized by spraying with 5% vanillin/ethanol solution followed by heating. Column chromatography (CC) was performed by gravity using glass columns of appropriate sizes with silica gel (230–400 mesh RM7484-500G, Himedia) or Sephadex LH-20 (Sigma–Aldrich).
Plant material
The was collected in Xuyen Moc district, Ba Ria–Vung Tau province, in June 2018. The scientific name of the plant was authenticated by M.Sc. Hoang Viet, Department of Ecology and Evolutionary Biology, Faculty of Biology, University of Science, Vietnam National University Ho Chi Minh City.
Extraction and isolation
Air-dried parts of (46 kg) were ground and extracted with methanol by the maceration method at room temperature. After filtering the solution, the solvent was evaporated under reduced pressure to obtain a crude methanolic extract (1.7 kg). This crude was used to prepare the -hexane extract using the liquid–liquid partition method. The -hexane extract (460 g) was subjected to silica gel column chromatography (CC) eluted with -hexane:ethyl acetate (stepwise, 99:1, 98:2, 95:5, 90:10, 50:50, 0:100, v/v) and ethyl acetate:methanol (stepwise, 99:1, 98:2, 95:5, 90:10, 50:50, 0:100, v/v) to afford 32 fractions (H1-H32). This paper reports the purification of three fractions, H2, H6, and H8. Fraction H2 (31.0 g) was divided into eight subfractions (H2.1-H2.8) by silica gel CC using the mobile phase -hexane:ethyl acetate (9:1, 8:2, 5:5, 0:10, v/v). Subfraction H2.8 (3 g) was repeatedly separated by preparative TLC to afford 1 (18 mg). Fraction H6 (8.28 g) was first chromatographed on Sephadex LH-20 to give ten subfractions (H6.1-H6.10). Subfraction H6.10 (900 mg) was subjected to silica gel CC eluted by -hexane:acetone (9:1) to afford 2 (5 mg). Fraction H8 (8.0 g) was subjected to silica gel CC and eluted by -hexane:acetone (9:1, 8:2, 5:5, 0:10) to give five fractions: H8.1 (93 mg), H8.2 (193 mg), H8.3 (1 g), H8.4 (698 mg), and H8.5 (2.7 g). Subfraction H8.4 (698 mg) was rechromatographed by silica gel CC and eluted with -hexane:chloroform (8:2) to afford two compounds 3 (7 mg) and 4 (13 mg). The same procedure was applied to fraction H8.5 (2.7 mg) eluted by -hexane:acetone (stepwise, 99:1, 98:2, 95:5) to obtain 5 (10 mg), 6 (4 mg) and 7 (20 mg), respectively.
RESULTS
The chemical investigation of the -hexane extract of Leonotis (L) R. Br led to the isolation ofseven compounds whose physical properties are presented in the following. The H and C-NMR data of compounds 1, 2, and 7 are presented in Tables 1 and 3, and compounds 3‒6 are reported in Tables 2 and 3.
-
(
E )- 10- Oxooctadeca- 8- enoic acid (1): White wax. ESI-MS/MS (negative mode)m/z 295.1 [M-H]- (calcd. for C18H32O3-H, 295.2). -
Ergosterol peroxide (2): White needles, mp 179–181 °C. LC–MSD (positive mode)
m/z 429.0 [M+H]+ (calcd. for C28H44O3+H, 429.3). -
Turmeronol A (3): Colorless oil. HR-ESI-MS (negative mode)
m/z 231.1386 [M-H]- (calcd. for C15H20O2-H, 231.1385). +248 (c 0.43, acetone). -
Methyl (
E )-3-(3,4-dimethoxyphenyl)propenoate (4): Colorless needles, mp. 69–70°C. HR-ESI-MS (positive mode) m/z 223.0963 [M+H]+ (calcd. for C12H14O4+H, 223.0970). -
Methyl 4-hydroxybenzoate (5): White crystalline solid, mp. 112–115
°C. HR-ESI-MS (negative mode) m/z 151.0397 [M-H]- (calcd. for C8H8O3-H, 151.0395). -
Methyl (
E )-3-(4-hydroxy-3-methoxyphenyl)propenoate (6): Colorless needles, mp. 64–65 °C. HR-ESI-MS (positive mode)m/z 207.0663 [M-H]- (calcd. for C11H12O4-H = 207.0657). -
Alpinumisoflavone (7): Pale yellow needles, mp. 210–213°C. HR-ESI-MS (negative mode)
m/z 335.0914 [M-H]- (calcd. for C20H16O5-H, 335.0919).

Chemical structures of seven isolated compounds
DISCUSSION
Compound 1 was obtained as white wax. The H-NMR spectrum of 1 displayed signals of two olefinic protons at 6.82 (1H, , 16.0, 6.5 Hz, H-8) and 6.08 (1H, , 15.5 Hz, H-9). The large coupling constants of 16.0 Hz of protons H-8 and H-9 suggested the -configuration of this double bond. The proton spectrum also showed a terminal methyl group [ 0.87 (3H, , 7.0 Hz, H-18)] and two methylene groups next to two carbonyl groups [ 2.51 (2H, , 7.0 Hz, H-11) and 2.44 (2H, , 7.0 Hz, H-2)]. The C-NMR spectrum (
The ESI-MS/MS spectrum of 1 (Figure 3) showed a pseudomolecular ion peak at 295.1 [M-H]. The MS/MS (negative mode) fragment patterns (Figure 3) suggested that 1 possessed a double bond at C-8, and this bond was adjacent to a ketone (C-10). These positions were supported by the HMBC experiment (Figure 2) with cross-peaks of protons H-8, H-9, H-11 to the carbon signal at 200.1 (conjugated ketone carbon, C-10) and of protons H-2 to the carbon signal at 169.7 (carboxyl carbon, C-1). The comparison of NMR and MS data of 1 with those of ()-10-oxooctadeca-8-enoic acid10, a synthetic compound obtained from the oxidation of oleic acid, showed good compatibility. Therefore, the chemical structure of 1 was suggested to be ()-10-oxooctadeca-8-enoic acid.
Compound 2 was isolated as white needles. The LC–MSD-MS spectrum showed a pseudomolecular ion peak at 429.0 [M+H] (calcd. for CHO+H, 429.3). The H-NMR spectrum of 2 showed signals of six methyl groups [ 1.00 (3H, , 7.0 Hz, H-21), 0.91 (3H, , 6.5 Hz, H-28), 0.83 (3H, , 7.0 Hz, H-27), 0.82 (3H, , 7.0 Hz, H-26), 0.82 (3H, , H-18) and 0.88 (3H, , H-19)], one oxygenated methine [ 3.97 (1H, , H-3)], two double bonds [ 5.15 (1H, , 15.5, 7.0 Hz, H-22),
The
|
No |
1 |
2 |
No |
7 |
|
2 |
2.44 ( |
2 |
7.81 ( | |
|
3 |
3.97 ( |
3 | ||
|
6 |
6.24 ( |
6 | ||
|
7 |
2.19 ( |
6.50 ( |
7 | |
|
8 |
6.82 ( |
8 |
6.34 ( | |
|
9 |
6.08 ( |
1.50 ( |
9 | |
|
11 |
2.51 ( |
1' | ||
|
12 |
2', 6' |
7.34 ( | ||
|
13 |
3', 5' |
6.83 ( | ||
|
15 |
3'' |
5.62 ( | ||
|
16 |
4'' |
6.73 ( | ||
|
18 |
0.87 ( |
0.82 ( |
2''-CH3 |
1.48 (6H, |
|
19 |
0.88 ( |
5-OH |
13.08 ( | |
|
20 | ||||
|
21 |
1.00 ( | |||
|
22 |
5.15 ( | |||
|
23 |
5.22 ( | |||
|
25 |
1.47 ( | |||
|
26 |
0.82 ( | |||
|
27 |
0.83 ( | |||
|
28 |
0.91 ( |
5.22 (1H, , 15.0, 7.5 Hz, H-23), 6.24 (1H, , 8.5 Hz, H-6) and 6.50 (1H, , 8.5 Hz, H-7)]. Its corresponding C-NMR spectrum showed 28 signals with four olefinic carbons [ 130.9 (C-7), 132.5 (C-23), 135.6 (C-6) and 135.4 (C-22)] and two oxygenated quaternary carbons [ 82.3 (C-5) and 79.6 (C-8)] of an ergosterol peroxide derivative. The good compatibility of its NMR and MS data with those of ergosterol peroxide in the literature11suggested that compound 2 was ergosterol peroxide.
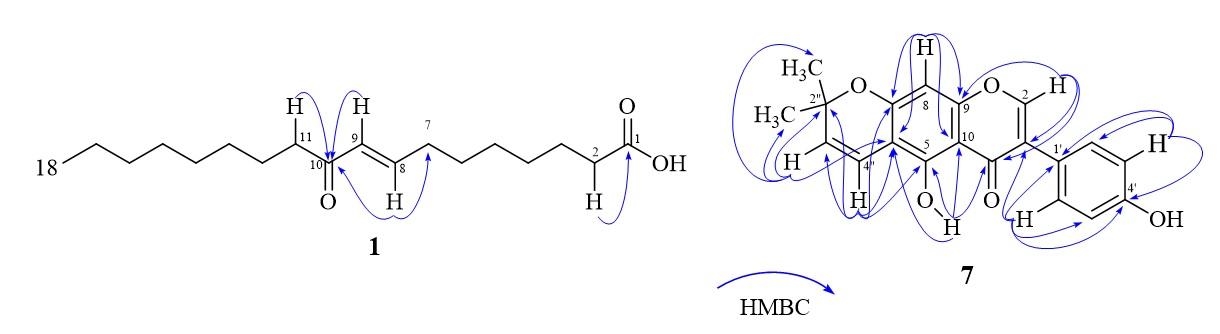
Keys HMBC of compounds 1 and 7

The (negative mode) MS/MS fragment patterns of compound 1
The
|
No |
3 |
4 |
5 |
6 |
|
2 |
6.66 ( |
7.04 ( |
7.95 ( |
7.02 ( |
|
3 |
6.88 ( | |||
|
5 |
7.02 ( |
6.86 ( |
6.88 ( |
6.92 ( |
|
6 |
6.70 ( |
7.10 ( |
7.95 ( |
7.07 ( |
|
7 |
3.24 ( |
7.63 ( |
7.62 ( | |
|
8 |
2.70 ( 2.59 ( |
6.30 ( |
6.29 ( | |
|
10 |
6.02 ( | |||
|
12 |
1.86 ( | |||
|
13 |
2.11 ( | |||
|
14 |
1.23 ( | |||
|
15 |
2.20 ( | |||
|
3-OCH3 |
3.90 ( |
3.92 ( | ||
|
4-OCH3 |
3.90 ( | |||
|
9-OCH3 |
3.80 ( |
3.79 ( | ||
|
7-OCH3 |
3.90 ( | |||
|
4-OH |
6.20 ( |
5.89 ( |
Compound 3 was isolated as a colorless oil. Its molecular formula was determined as CHO through its pseudomolecular ion peak at 231.1386 [M-H] (calcd. for CHO-H, 231.1385). The H-NMR spectrum (
Compound 4 was obtained as colorless needles. Compound 4 possessed a 1,3,4-trisubstituted benzene ring with proton NMR signalsat 7.10 (1H, , 8.0, 2.0 Hz, H-6), 7.04 (1H, , 1.5 Hz, H-2), 6.86 (1H, , 8.5 Hz, H-5). The H-NMR spectrum also showed a six-proton singlet at 3.90 (6H, ) for the two methoxy groups and two olefinic protons at 7.63 (1H, , 16.0 Hz, H-7), 6.30 (1H, , 16.0 Hz, H-8) of an -configuration double bond. The C-NMR spectrum of 4showed signals of one carboxyl carbon ( 167.8, C-9), two olefinic carbons ( 144.9, C-7 and 115.7, C-8), six aromatic carbons [ 151.4 (C-4), 149.5 (C-3), 127.6 (C-1), 122.7 (C-6), 111.3 (C-5), 110.0 (C-2)] and three methoxy groups [ 56.1 (3-OCH and 4-OCH) and 51.7 (9-OCH)]. In addition, the HR-ESI-MS spectrum of compound 4 showed a pseudomolecular ion peak at 223.0963 [M+H] (calcd. for CHO+H, 223.0970).
13C-NMR (CDCl3, 125 MHz) data of compound 1‒7
|
No |
1 |
2 |
3 |
4 |
5 |
6 |
No |
7 | |
|
1 |
169.7 |
34.9 |
146.2 |
127.6 |
122.7 |
127.2 |
2 |
152.8 | |
|
2 |
35.3 |
30.3 |
113.7 |
110.0 |
132.1 |
109.6 |
3 |
123.8 | |
|
3 |
24.3 |
66.6 |
155.4 |
149.5 |
115.4 |
148.2 |
4 |
181.2 | |
|
4 |
28.0 |
37.1 |
121.5 |
151.4 |
160.3 |
147.0 |
5 |
157.0 | |
|
5 |
29.5 |
82.3 |
131.1 |
111.3 |
115.4 |
115.4 |
6 |
105.8 | |
|
6 |
29.6 |
135.6 |
119.1 |
122.7 |
132.1 |
123.2 |
7 |
159.8 | |
|
7 |
32.60 |
130.9 |
35.5 |
144.9 |
167.4 |
145.1 |
8 |
95.1 | |
|
8 |
146.9 |
79.6 |
52.8 |
115.7 |
114.9 |
9 |
157.5 | ||
|
9 |
130.5 |
51.3 |
200.1 |
167.8 |
167.8 |
10 |
106.3 | ||
|
3-OCH3 |
56.1 |
56.1 |
1' |
123.0 | |||||
|
4-OCH3 |
56.1 |
2' |
130.5 | ||||||
|
9-OCH3 |
51.7 |
51.7 |
3' |
115.9 | |||||
|
7-OCH3 |
52.1 |
4' |
156.2 | ||||||
|
10 |
200.1 |
37.1 |
124.3 |
5' |
115.9 | ||||
|
11 |
40.3 |
23.6 |
154.0 |
6' |
130.5 | ||||
|
12 |
24.5 |
39.5 |
27.8 |
2'' |
78.3 | ||||
|
13 |
28.3 |
44.7 |
20.9 |
3'' |
128.4 | ||||
|
14 |
29.3 |
51.9 |
22.1 |
4'' |
115.6 | ||||
|
15 |
29.2 |
20.8 |
15.5 |
2''-CH3 |
28.5 | ||||
|
16 |
32.0 |
28.8 | |||||||
|
17 |
22.8 |
56.4 | |||||||
|
18 |
14.2 |
13.0 | |||||||
|
19 |
18.3 | ||||||||
|
20 |
39.9 | ||||||||
|
21 |
21.0 | ||||||||
|
22 |
135.4 | ||||||||
|
23 |
132.5 | ||||||||
|
24 |
42.9 | ||||||||
|
25 |
33.2 | ||||||||
|
26 |
19.8 | ||||||||
|
27 |
20.1 | ||||||||
|
28 |
17.7 |
The comparison of these HR-MS and NMR data of 4with those of methyl ()-3-(3,4-dimethoxyphenyl)acrylate in the literature14 showed goodmethyl ()-3-(3,4-dimethoxyphenyl)acrylate with the systematic name methyl ()-3-(3,4-dimethoxyphenyl)propenoate.
Compound 6 was obtained as colorless needles. The molecular formula was determined to be CHOthrough its pseudomolecular ion peak at 193.0501 [M+H] (calcd. for CHO+H, 193.0864). The similarity in the NMR data (Tables 2 and 3) of 6 and 4 with just one difference of the replacement of the methoxy group of the benzene ring in 4 [ 3.90 (3H, , 4-OCH)] by a hydroxy group in 6 [ 5.89 (1H, , 4-OH)] suggested that the latter wasmethyl ferulate15 or its systematic name,methyl ()-3-(4-hydroxy-3-methoxyphenyl)propenoate.
Compound 5 was isolated as a white crystalline solid. Its MS spectrum showed a deprotonated molecular ion peak at 151.0397 [M-H] (calcd. for CHO-H, 151.0395). The H-NMR spectrum (
Compound 7 was obtained as pale yellow needles. Its H-NMR spectrum showed proton signals at 13.08 (1H, 5-OH), 7.81 (1H, , H-2), 7.34 (2H, , 8.5 Hz, H-2', H-6'), 6.83 (2H, , 8.5 Hz, H-3', H-5'), 6.73 (1H, , 10.5 Hz, H-4''), 6.34 (1H, , H-8), 5.62 (1H, , 10.0 Hz, H-3'') and 1.48 (6H, , two methyl groups, 2''-CH). The proton NMR data of 7 were almost identical to those of 4'--methylalpinumisoflavone, a compound previously isolated from a less polar H5 fraction, with one difference of lacking the methoxy signal at 3.84 in the former17. The comparison of the C-NMR data of these two compounds as well as that of alpumisoflavone18showed good compatibility. This was further supported by the HR-ESI-MS spectrum with a deprotonated molecular ion peak at 335.0914 [M-H] (calcd. for CHO-H, 335.0919) and by all appropriate cross-peaks in the HMBC spectrum (Figure 2). Therefore, the chemical structure of 7 was proposed as alpumisoflavone.
CONCLUSION
Further chemical studies on the -hexane extract of Leonotis nepetifolia (L.) R. Br, collected at Xuyen Moc district, Ba Ria–Vung Tau province, using some column chromatographic separations, seven compounds were isolated. Their structures were elucidated as 10-oxooctadeca-8-enoic acid (1), ergosterol peroxide (2), turmeronol A (3), methyl ()-3-(3,4-dimethoxyphenyl)propenoate (4), methyl 4-hydroxybenzoate (5), methyl ()-3-(4-hydroxy-3-methoxyphenyl)propenoate (6), and alpinumisoflavone (7) Although these compounds are known in other species, this is the first time they have been reported in .
ABREVIATIONS
LC–MSD: Liquid chromatograph/mass selective detector
LC–MS/MS: Liquid chromatography with tandem mass spectrometry
HR-ESI-MS: High resolution electrospray ionization-mass spectrometry
H NMR: Proton Nuclear Magnetic Resonance
CNMR: Carbon-13 Nuclear Magnetic Resonance
HMBC: Heteronuclear Multiple Bond Correlation
: singlet
: doublet
: doublet of doublets
: triplet
: multiplet
COMPETING INTEREST
The authors declare no competing financial interest.
AUTHORS’ CONTRIBUTION
Nguyen T.K.H., Phan T.T., Le N.H.T. interpreted NMR and MS data and searched the bibliography. Nguyen K.P.P., Ngo T.T.D. contributed to conducting experiments and acquiring MS and NMR data and gave the final correction for the manuscript.
Corresponding author: Nguyen Thi Kim Huong, University of Science, National University-Ho Chi Minh City, . Email: kimhuong241@gmail.com
ACKNOWLEDGMENTS
This research was funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number B 2019-18-05.

