Application of Antioxidant in BOPP book cover recycling
- Polymer Chemistry Department, University of Science, 227 Nguyen Van Cu Street, District 5, Ho Chi Minh City, Vietnam National University Ho Chi Minh City, Viet Nam
Abstract
BOPP (biaxial oriented polypropylene) thin films are commonly used as book covers at schools in Vietnam. At the end of every academic year, a large quantity of BOPP book covers become waste. Therefore, the selective collection and recycling of this material are necessary. The plastic material was separated from paper directly by students from the school, and then it was shredded into small pieces and extruded into pellets with and without antioxidants. The composition of the commercial antioxidant was confirmed by FTIR analysis as a blend of 1:2 by weight of Irganox1010/Irgafos 168. The melt index test showed that after thermal aging of recycled PP pellets at 200 ◦C, they were strongly degraded. However, the addition of antioxidants to the recycled PP samples effectively protected them from degradation. The melt viscosity of recycled PP followed by a mini twin screw extruder also confirmed the protective role of the antioxidant. The larger the quantity of antioxidant used, the more stable the torque or melt viscosity of PP became. The optimal usage of 1% Irganox B 215 can protect PP from further degradation.
INTRODUCTION
Due to its high moisture resistance, optical clarity and tensile strength, biaxial oriented polypropylene (BOPP) is commonly used in various applications, such as packaging, adhesive tape, and stationery.
For the academic year of 2021-2022, in Ho Chi Minh City, 1.71 million students were enrolled1. In most schools, books and notebooks of students are required to be covered with plastic sheets, mostly made from BOPP. A student needs at least 16 books per year, and one sheet of plastic cover weights approximately 8 grams. Then, we can determine that for one year, the total plastic waste could be estimated as 16 books/year x 8 g/book cover x 1.71x10 students = 219 tons/year!
After usage, the plastic book covers were usually mixed with paper as a waste material. However, the recycling process requires separating plastics from paper. In our project, this separation step can be performed by students themselves. At the end of an academic year, by cooperation with teachers, we can obtain only plastic materials. Additionally, in this way, we can help students understand that the first step of recycling is the effective separation of various materials.
Plastics are normally degraded during processing and usage therefore, stabilizers and impact modifiers are added in the reprocessing step, especially in the mechanical recycling process. The reprocessed pellets can be used for nonfood contact products, namely, pots for trees, plastic building materials, and outdoor furniture.
The mechanical or physical recycling of PP has long been investigated 2, 3, 4, 5. The mechanical properties of the recycled PP were improved by adding antioxidants (AO), impact modifiers, and compatibilizers. Phenolic antioxidants were investigated6, 7, 8, 9, and the results showed that antioxidants can effectively retard thermal degradation.
Irganox 1010 (BASF)/Hostanox 010 (Hoechst) is a primary antioxidant containing four sterically hindered phenolic groups (Figure 1a) with the chemical name pentaerythritol tetrakis[3-(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionate] (CAS 6683-19-8, MW 1177,6 g/mol). This is a H-donor, and it plays the role of a radical scavenger.
Irgafos 168 (BASF)/Hostanox PAR 24 (Hoechst) or tris(2,4-di-tert-butylphenyl) phosphite (CAS 31570-04-4, MW 646,9 g/mol) is a secondary phosphite antioxidant (Figure 1b). This antioxidant can protect polymers by reacting with hydroperoxides formed by polymer oxidation therefore, it can prevent induced degradation and extend the performance of primary antioxidants.

Molecular structures of (a) Irganox 1010 (BASF) and (b) Irgafos 168 (BASF)
Irgafos 168 performs effectively when combined with Irganox 1010. Some blends of Irganox 1010 with Irgafos 168 were commercialized, such as Irganox B 561, Irganox B 215, and Irganox B 225.
Commercial blends of Irganox 1010 with Irgafos 168
|
Irganox 1010:Irgafos 168 |
Irganox B 561 |
Irganox B 215 |
Irganox B 225 |
|---|---|---|---|
|
Weight ratio |
1:4 |
1:2 |
1:1 |
|
Molar ratio |
1:7.28 |
1.3.64 |
1:1.82 |
Until now, there has been no report on the mechanical recycling of BOPP book covers. In this research project, we converted BOPP thin films into pellets by using an extruder. During this reprocessing step, antioxidant was added to protect them from thermal degradation. The composition of the commercial antioxidant was confirmed by FTIR and DSC. The stability of the obtained pellets was monitored by melt index values, and the change in melt viscosity was followed by a mini twin screw extruder. From these experimental data, we can determine the optimal quantity of antioxidant used for BOPP film recycling.
EXPERIMATION
Materials
Book cover films were collected, separated from papers, and labeled (Figure 2). The weights of book cover films for book sizes of 21x16 and 25x17 cm are 7.56 and 9.10 g, respectively. Antioxidants were donated from industry. The structures of waste book cover films and commercial antioxidants were confirmed by FTIR and DSC analysis.

Notebooks with plastic covers (left) and used plastic covers.
Instruments
The lab-scale single-screw extruder has a length/diameter ratio of 25/1, with L = 80 cm and D = 32 mm. Plastic extrusion was performed with a screw speed of 6 rpm. The temperature of the barrel and die was set as 190 °C. The thermal properties of the materials were characterized by differential scanning calorimetry (DSC) experiments using a METTLER STARe SW 11.00 instrument (Mettler-Toledo Ltd.).
The melt flow index (MFI) of the plastic material was determined by using a Tinius Olsen Extrusion Plastometer. A standard load of 2.16 kg was applied on PP at a die temperature of 230 °C, and the flow of material was observed for 10 min (ASTM D1238).
The melt viscosity or torque of the plastic materials was measured by using Thermo Scientific HAAKE MiniLab II equipment by heating at 200 °C and at a screw speed of 50 rpm. The system is based on a conical counter twin-screw compounder with an integrated recirculation channel. A sample amount of 5 g was used. The torque of the drive motor was monitored.
Preparation samples for testing
BOPP book covers were collected from some schools and then shredded into small pieces of 5x5 cm. These small films were melted and mixed with 0.5, 1.0 and 1.5% AO by a lab-scale single-screw extruder. The cooled PP strands were cut into 2-3 mm pellets.
The thermal aging of PP pellets was performed in air at different temperatures on a hot plate. The temperature of the hot plate surface was set and measured by an infrared thermometer, and then 10 g of PP in a petri dish was left for 3 hrs. At 200 °C, the PP sample was strongly degraded, and then the heating time was reduced to 0.5 h.
RESULTS
Characterization of materials
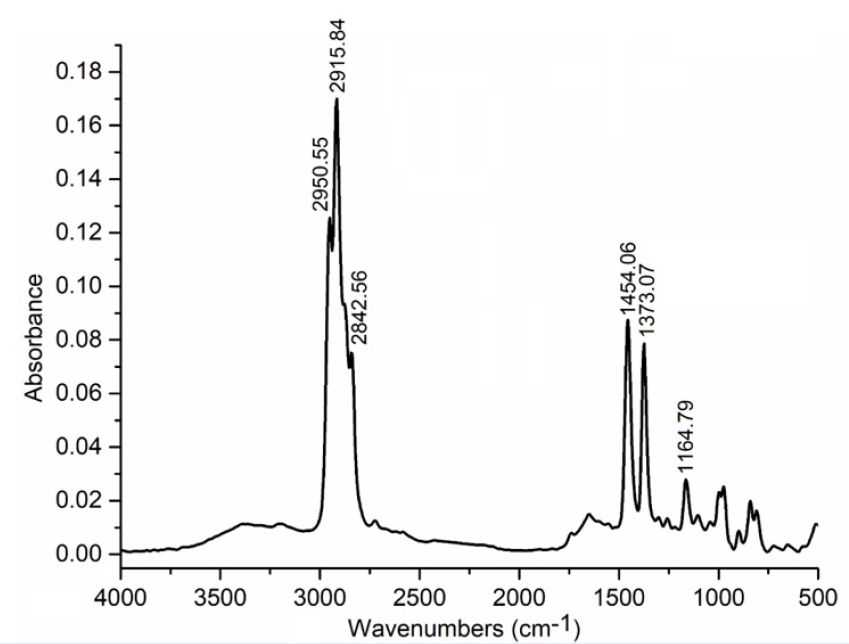
Figure 3 shows the FTIR spectrum of recycled PP.

FTIR of book cover plastic film
The FTIR reference spectra of Irganox1010, Irgafos 168, and Irganox B 561/B 215/B 225 are compared and given in Figure 4. From the FTIR spectra, a peak at 1740 cm was selected as representative for Irganox1010, and another peak at 1491 cm was chosen as indicative of Irgafos 168. The data were analyzed (

FTIR reference spectra of (a) Irganox 1010, (b) Irgafos 168, (c) Irganox B 561 (1:4), (d) Irganox B 215 (1:2), and (e) Irganox B 225 (1:1)[https://spectrabase.com]
Peak ratio (PR) of I(1740 cm-1)/I(1491 cm-1) of three Irganox samples from the reference spectra
|
Trade name |
Weight Ratio of Irganox1010/Irgafos 168 |
Peak ratio (PR) of I(1740 cm-1)/I(1491 cm-1) |
Log(PR) |
|---|---|---|---|
|
Irganox B 561 |
1/4 or 0.25 |
0.210 |
-0.678 |
|
Irganox B 215 |
1/2 or 0.50 |
0.375 |
-0.426 |
|
Irganox B 225 |
1/1 or 1.00 |
1.478 |
0.170 |

The graph of weight ratio (WR) of Irganox1010/Irgafos 168 versus peak ratio (PR) of I(1740 cm-1)/I(1491 cm-1) (above) and Log(PR) (below)
The FTIR spectrum of the commercial antioxidant is given in Figure 6. From the peak ratio (PR) I(1740 cm)/I(1491 cm) of this spectrum, the weight ratio of antioxidants was calculated.

FTIR of the commercial antioxidant
The melting process of the commercial antioxidant was observed by DSC experiments. The sample was heated from room temperature to 250 °C at a heating rate of 10 °C/m in a nitrogen atmosphere. The DSC curve is shown in Figure 7.

DSC of the commercial antioxidant Irganox B215
Melt flow index (MFI) testing after thermal treatment
MFI testing experiments were performed for rPP pellets without the addition of AO during extrusion in the first step after heating at various temperatures on a hot plate for 3 h, except for only 0.5 h for the sample at 200 °C. The obtained data are shown in Figure 8.

MFI of rPP without AO after heating at various temperatures for 3 h, except 0.5 h at 200 °C
Various contents of AO were added during extrusion, and then the obtained pellets were heated at 200 °C for 30 min. After cooling to room temperature, the MFI values of the samples were measured and are given in Figure 9.

MFI of rPP samples with various Irganox B 215 contents before and after thermal treatment at 200 °C for 30 m
Extruder torque results from Thermo Scientific HAAKE MiniLab II equipment
The resistance of the drive motor of an extruder because of the melt viscosity of polymers is expressed as the extruder torque. The torque values (Figure 10, Figure 11) of the rPP pellets with different Irganox B 215 contents and virgin PP were obtained by using a Thermo Scientific HAAKE MiniLab II extruder at 200 °C and 50 rpm.

The overall torque curves of virgin PP and recycled PP with different Irganox B 215 contents
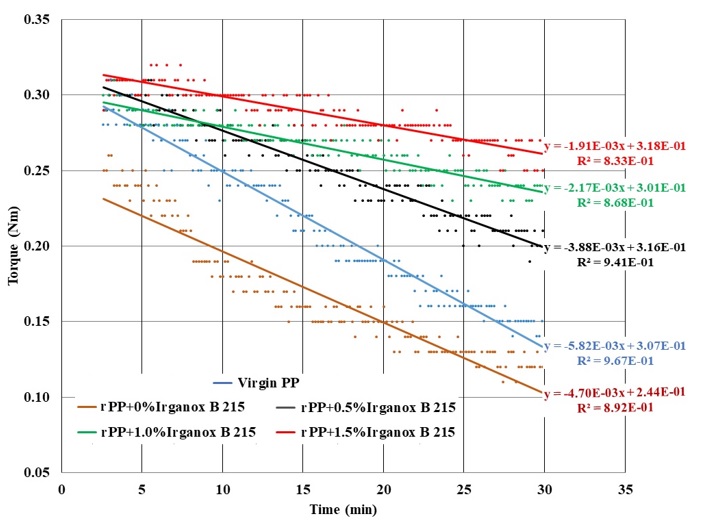
The extract graph from 2.5 to 30 m of torque curves of virgin PP and recycled PP with different Irganox B 215 contents
The extruder torque reduction rates were considered as the slopes of the linear relationships between torque and time at 200 °C of various samples. The data are shown in
Torque reduction rates (Nm/minx103) at the melt state (200 °C) of PP samples
|
Virgin PP |
rPP+0% AO |
rPP+0.5% AO |
rPP+1.0% AO |
rPP+1.5% AO | |
|
Torque reduction rates |
-5.82 |
-4.70 |
-3.88 |
-2.17 |
-1.91 |
Thermal characterization of PP by DSC
The thermal properties of the PP samples were investigated by DSC. The samples were heated from 25 to 220 °C and then cooled back to 25 °C at heating/cooling rates of 10 °C/m. The DSC curves of vPP and rPP are given in Figure 12.
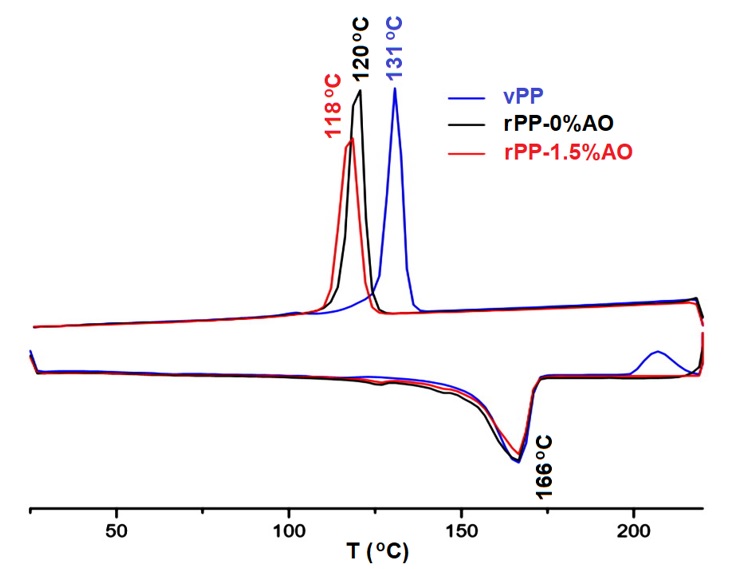
DSC heating and cooling curves of vPP, rPP-0%AO, and rPP-1.5%AO samples
Data extracted from the DSC experiments of vPP, rPP-0%AO, and rPP-1.5%AO samples
|
vPP |
rPP-0%AO |
rPP-1.5%AO | |
|---|---|---|---|
|
Tm (at peak) (°C) |
166.18 |
166.35 |
166.70 |
|
|
81.81 |
64.71 |
71.02 |
|
Tc (at peak) (°C) |
131.03 |
119.72 |
117.73 |
|
|
-90.50 |
-90.51 |
-86.99 |
DISCUSSION
Characterization of materials
The BOPP film was first characterized by FTIR. The spectrum in Figure 3 shows typical peaks of CH stretching and bending vibrations at 2951-2843 and 1454-1373 cm, respectively.
Various commercial AOs have been used for plastics. The brand names of BASF’s AO are Irganox or Hoechst’s AO are Hostanox, and many other names of the same hindered phenolic compounds, namely, Ralox 630, Antioxidant 1010, Primanox, etc. The complexity of the AO in the market requires analytical methods to identify the structure and composition. Here, we used FTIR spectroscopy as a versatile method.
The ester groups of Irganox 1010 show a medium band at 1740 cm of C=O stretching (Figure 4). The vibration of the isolated OH of phenol appears as a doublet at 3639/3599 cm. The phosphite structure of Irgafos 168 was confirmed by the presence of signals at 1195/1212, 1082, 854 and 775 cm. Both OAs have t-butyl groups that give 2 bands at 1396 and 1362 cm of symmetrical CH deformation vibration. The band at 1491 cm is due to the asymmetrical deformation vibration of CH.
The comparison of reference spectra of Irganox1010, Irgafos 168, and Irganox B 561/B 215/B 225 is given in Figure 4. The reported weight ratios of Irganox1010/Irgafos 168 in Irganox B 561/B 215/B 225 are 1/4, 1/2, and 1/1.
In the spectrum of the blends, if we use the signal at 1740 cm as a representative peak for Irganox 1010 and 1491 cm for Irgafos 168, then the ratio of peak intensities PR = I(1740 cm)/I(1491 cm) was calculated.
The graph of the weight ratio (WR) of Irganox1010/Irgafos 168 versus the peak ratio (PR) of I(1740 cm)/I(1491 cm) gave a logarithmic relationship WR = 0.381xLn(PR) + 0.8563 with R = 0.9984 (Figure 5). If a Log(PR) function was used in place of PR, then a linear relationship was obtained as WR = 0.8763Log(PR) + 0.8563 (Figure 5).
From the FTIR spectrum of the commercial sample (Figure 6), we can determine the peak ratio (PR) of I(1740 cm)/I(1491 cm) as 0.3455, and from the above logarithmic or linear relationship, we can determine y = 0.452 ~ 0.5 or the antioxidant must be Irganox B 215.
The DSC of Irganox B 215 (Figure 7) shows two separated melting endo peaks at 117.13 °C and at 181.47 °C of Irganox 1010 and Irgafos 168, respectively. Djouani et al. 10 also reported the melting temperatures of these antioxidants at 117.6 °C and 185.5 °C. Here, again, DSC characterization confirmed Irganox B 215 as a blend of Irganox 1010 and Irgafos 168.
Melt flow index (MFI) testing after thermal treatment
Thermal aging of polymers is a long-term, irreversible change in their structures, composition, and morphology by heating. The most important chemical change is due to the reaction with atmospheric oxygen by a free radical mechanism. In particular, the PP chain has tertiary hydrogens that are very sensitive to oxidative degradation. Therefore, AO should be added to polymers to protect them.
The melt flow index (MFI) or melt flow rate (MFR) is used as a typical indicator of polymer degradation. After heating the rPP pellets at various temperatures on a hot plate for 3 h, except for only 0.5 h for the sample at 200 °C, MFI testing experiments were performed. The obtained data are shown in Figure 8. This graph showed that rPP was rather stable below 170 °C for 3 hrs. However, it degraded quickly while heating at 200 °C for just 0.5 h, leading to a reduction in viscosity and then an MFI increase. Consequently, 200 °C was selected as the temperature to test the protective effect of AO.
The MFI values of the samples with various contents of AO after heating at 200 °C for 30 m were measured and are given in Figure 9. The graph clearly showed that at a content of more than 1%, Irganox B 215 was very effective in protecting rPP from thermal degradation. The MFIs of the rPP samples with 1.0 and 1.5% Irganox B 215 are almost equal to the MFI of virgin PP of 4.79 g/10 m. Consequently, to reduce the cost of recycling, the addition of AO in the rPP should not be higher than 1%.
Extruder torque results from Thermo Scientific HAAKE MiniLab II equipment
The rPP sample without AO showed the lowest maximum torque due to the thermal degradation in the pellet formation step. Other samples showed similar maximum torque values or rather higher values than the virgin PP. The virgin PP sample after reaching the maximum torque degraded much faster than the rPP with AO. When a larger quantity of AO was added, the extruder torque decreased more slowly. This result confirmed that rPP was efficiently protected even better than virgin PP without the addition of AO.
The prolonged time at the melt state (Figure 11) showed that the torque or viscosity values of the various systems decreased in the order of (rPP+1.5 Irganox B 215) > (rPP+1.0 Irganox B 215) > (rPP+0.5 Irganox B 215) > Virgin PP > (rPP+0 Irganox B 215). These results are good proof for the protection capability of the AO system in long-time extrusion. In addition, the higher the Irganox B 215 content was added, the lower the reduction rate of torque was observed (
Thermal characterization of PP by DSC
The heating curves (Figure 12) and the data in
While cooling, PP samples show exo crystallization peaks. Virgin PP has a higher purity and is readily recrystallized at a higher temperature than rPP samples. The lower recrystallization temperature of rPP required a longer cooling time for rPP processing, therefore lowering the processing output. This is a detriment in using rPP in the injection molding process. Nucleating agents are normally used to solve this problem. However, the application of nucleating agents in rPP is not included in the objective of this research project.
CONCLUSION
The structures and compositions of commercial AO were identified for the first time simply by FTIR spectroscopy. A linear relationship between weight ratio (WR) and log(peak ratio) (PR) was found as WR = 0.8763Log(PR) + 0.8563. The DSC of Irganox B215 shows two melting peaks at 117.13 °C and at 181.47 °C of Irganox 1010 and Irgafos 168, respectively. The melting ranges of this AO are matched with the processing temperature of PP.
BOPP films were collected from some schools in Ho Chi Minh City. After being shredded into small sizes, they were extruded with the addition of Irganox B 215 from 0.5, 1.0 to 1.5%. The degradation of PP pellets was followed by MFI testing of samples after thermal aging on a hot plate at various temperatures. The PP samples quickly degraded at 200 °C.
The melt viscosity or torque values obtained from experiments using a Thermo Scientific HAAKE MiniLab II extruder showed that the larger the quantity of AO used, the slower the torque reduction, which means that rPP was efficiently protected even better than virgin PP without AO. However, the content of 1% Irganox B 215 should be an optimal value for protection effects and for the cost of reprocessing.
Abbreviations
AO: Antioxidant
BOPP: Biaxial oriented polypropylene
DSC: Differential scanning calorimetry
FTIR: Fourier-transform infrared
MFI/MFR: Melt flow index/Melt flow rate
PP: Polypropylene
PR: Peak ratio
rPP: Recycled polypropylene
vPP: Virgin polypropylene
WR: Weight ratio
Acknowlegdement
This research is funded by the University of Science, VNU-HCM under grant number HH 2021-05.
Author’s contributions
Hoang Ngoc Cuong designed the research, interpreted experimental data, wrote the original draft, reviewed, and edited the manuscript.
Le Thi Hoa Xuan Nguyet performed the experiments.
Conflict of interest
The authors declare that they have no competing interests.

