Synthesis and Optical Properties of Conjugated Copolymers based on Phenoxazine and Fluorene for an Activated Layer in Polymeric Solar Cell Applications
- Faculty of Chemical Engineering, Ho Chi Minh City University of Technology, Vietnam National University, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam
- National Key Laboratory of Polymer and Composite Materials– Ho Chi Minh City, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam
- Institute of Applied Materials Science, Vietnam Academy of Science and Technology, Ho Chi Minh City, 71515, Vietnam
- Faculty of Materials Technology, Ho Chi Minh City University of Technology, Vietnam National University, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam
Abstract
In this research, a conjugated copolymer based on phenoxazine and fluorene units was synthesized via palladium-catalyzed Suzuki–Miyaura coupling polymerization in the presence of a Pd catalyst and K2CO3 as the base. The number-average molecular weight of conjugated polymers was obtained to be approximately 2616 g/mol with a polydispersity index of 1.65. The yield of polymerization was established to be approximately 29%. The chemical structure, average molecular weights and optical properties of the conjugated polymer were characterized via proton nuclear magnetic resonance (1H NMR) and Fourier transform infrared (FT-IR) spectroscopies, gel permeation chromatography (GPC), UV−vis and fluorescence spectroscopies. The conjugated polymer showed an optical band gap of 2.09 eV. The obtained conjugated polymers displayed fluorescence properties under an excitation wavelength of 400 nm. The conjugated polymers exhibit potential as activated layers in organic solar cells, proving the analysis results.
Introduction
In recent years, conjugated polymers have attracted much attention for their applications in many soft electronic applications, such as the efficient hole/electron transport layer in organic solar cells (OSCs), organic field effect transistors (OFETs), organic light emitting diodes (OLEDs), and electrochromic devices (EC), due to their good solubility, thermal stability, and large area processing.1, 2, 3, 4, 5 Among the building units for the synthesis of conjugated polymers, phenoxazine as well as fluorene and its derivatives have been extensively used in conjugated polymers for hole-transporting polymer layers in photoelectronic applications, including OSCs, OLEDs, and EC devices.6, 7, 8, 9, 10, 11 Jinqing Qu also reported the synthesis of conjugated copolymers via Sonogashira coupling polycondensation of -octadecyl- and -octyl-3,6-diethynylcarbazoles with tris(4-iodophenyl)amine and 1,3,5-tribromobenzene. The obtained polymers have a number average molecular weight (M) in the range of 3.5500 – 21000 g.mol and demonstrate that the polymers are electronically redox-active, which can be applied for tunable light-emitting materials based on carbazole-containing polymers.12 In addition, the conjugated polymers based on phenoxazine also exhibited a narrow band gap and redshift absorption and were in the oxidized state owing to the electron-rich N atom in their structure.13, 14 Marri Anil Reddy reported the synthesis and fabrication of organic solar cells based on phenothiazine and phenoxazine moieties with PCE up to 6%.12, 15, 16, 17 Interestingly, Haining Tian synthesized small molecules of phenoxazine chromophores based on triphenylamine with phenoxazine containing vinyl and CN end groups that were used in organic dye-sensitized solar cells with PCE of 7.7%.14 Jenekhe’s group fabricated OFETs based on the alternating phenoxazine-fluorene copolymer (POF2) as a p-channel semiconductor with a hole mobility of 3 x 10 cm/(V s).18, 19 More recently, Jong Hyuk Bae synthesized phenoxazine dyes with a hexyloxy chain and furan moiety and used them in dye-sensitized solar cells that showed the best conversion efficiency of 6.34%.20 It is worth noting that a phenoxazine moiety includes electron-rich oxygen and nitrogen atoms in a heterocyclic ring and displays high electron-donating capability therefore, it also has suitability as a building moiety for promising organic solar cell applications. However, conjugated polymers based on (4-hexylphenyl)(10H-phenoxazin-10-yl)methanone and fluorene moieties have not been reported. In addition, conjugated polymers containing (4-hexylphenyl)(10H-phenoxazin-10-yl)methanone and fluorene are expected to be electron-rich donor materials that can enhance the conversion efficiency of organic solar cells.
In this research, new conjugated polymers based on phenoxazine derivatives and fluorene units were designed and synthesized via palladium-catalyzed Suzuki–Miyaura coupling polymerization. The obtained conjugated polymer will be investigated for the optical properties to find reasonable bandgaps for organic solar cell applications.
Experiment
Materials
10H-phenoxazine (98%) and fluorene were purchased from Ark Pharm (USA). Triphenylamine, benzo [c] [1, 2, 5] thiadiazole, tetrahydrofuran (99.9%) and -bromosuccinimide (NBS) were purchased from Acros Organics. 2-Ethylhexyl bromide (95%), palladium(II) acetate (Pd(OAc), 98%), tricyclohexylphosphine tetrafluoroborate (PCy·HBF, 97%), 3,3′dibromo-2,2′bithiophene and pivalic acid (PivOH, 99%) were purchased from Sigma‒Aldrich/Kantochem and used as received. Potassium carbonate (KCO, 99%) and sodium chloride (NaCl, 99%) were purchased from Acros/Merck. Chloroform (CHCl, 99.5%) and dimethylacetamide (DMAc, 99%) were purchased from Fisher/Acros and dried using molecular sieves under N. Dichloromethane (99.8%), -hexane (99%), methanol (99.8%) and ethyl acetate (99%) were purchased from Fisher/Acros and used as received.
Characterization
H NMR spectra were recorded in deuterated chloroform (CDCl) with TMS as a standard on a Bruker Avance 500 MHz. Fourier transform infrared (FT-IR) spectra, collected as the average of 264 scans with a resolution of 4 cm, were recorded from KBr disks on the FT-IR Bruker Tensor 27. Gel permeation chromatography (GPC) measurements were performed on a Polymer PL-GPC 50 gel permeation chromatograph system equipped with an RI detector, with tetrahydrofuran as the eluent (flow rate: 1.0 ml/min). The molecular weight and molecular weight distribution were calculated with reference to polystyrene standards. The UV‒Vis and fluorescence spectra were characterized using an Ocean Optics instrument. The yield of all reactions was calculated by averaging the number of experiments.
Synthesis of the (4-hexylphenyl)(10H-phenoxazin-10-yl)methanone (HP-POzM) monomer
10H-phenoxazine (300 mg, 1.64 mmol) was dissolved in THF (4 mL) in a 100 mL flask under nitrogen, followed by stirring for several minutes. Then, the compound was cooled to 0 °C. 4-Hexylindobenzoyl chloride (368.56 mg, 1.64 mmol) and triethylamine (TEA) (200 mg, 1.97 mmol) were added, and the mixture was degassed via a free-pump-thaw cycle three times. Then, the reaction was carried out at 65 °C for 72 h. After completion of the reaction, the compound was extracted with 50 mL of CHCl, washed with distilled water, and dried over anhydrous KCO. The mixture was evaporated to obtain the crude product, which was purified by silica column chromatography with acetate/hexane (1:50, v/v) and dried under vacuum at 50 °C for 24 h to obtain the compound as a yellow oil (354,72 mg, 58.3%).
Synthesis of the (3,7-dibromo-10H-phenoxazin-10-yl)(4-hexylphenyl)methanone (DBrHP-POzM) monomer
HP-POzM (100 mg) was added to 5 ml of DMF in a 00 mL flask under nitrogen, followed by stirring for several minutes. The mixture was cooled to 0 °C, and the flask was covered with aluminum wrap to prevent visible light. Then, 106 mg of -bromosuccinimide (NBS) (0.594 mmol) was dissolved in 5 ml DMF that was added dropwise into the reaction. The mixture was incubated for 24 hours. After completion of the reaction, the compound was extracted with 50 mL of CHCl, washed with distilled water, and dried over anhydrous KCO. The mixture was evaporated to obtain the crude product, which was purified by silica column chromatography DCM/hexane (1:1, v/v) and dried under oven vacuum at 50 °C for 24 hours.
Synthesis of polymer (4-hexylphenyl)(3-methyl-7-(7-methyl-9,9-dioctyl-9H-fluoren-2-yl)-10H-phenoxazin-10-yl)methanone (PO1)
(3,7-Dibromo-10H-phenoxazin-10-yl)(4-hexylphenyl)methanone (106 mg, 0,2 mmol) and 2,2'-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis[4,4,5,5-tetramethyl-1,3,2-dioxaborolan (128.5 mg, 0.2 mmol) were dissolved in 10 mL DMAc in a 100 mL flask under nitrogen. Then, Pd(PPh) (23.2 mg, 0.02 mmol) and KCO (138.2 mg, 1 mmol) were added to the flask, and the solution was heated at 100 °C and stirred for 72 h. Then, the mixture was cooled to room temperature, and the polymer was precipitated by the addition of 50 ml of methanol and filtered through a Soxhlet thimble, which was then subjected to Soxhlet extraction with methanol, -hexane, and chloroform. Next, the resulting solution from the chloroform fraction was precipitated in 50 ml of methanol. The polymer was recovered as a grayish solid sample by filtration and dried under vacuum at 50 °C for 24 h to obtain the final product PO1 (45 mg, yield 29%).
Results
The ((4-hexylphenyl)(10H-phenoxazin-10-yl)methanone monomer was synthesized via a C-N coupling reaction between 10H-phenoxazine and 4-hexylbenzoyl chloride. Figure 1 showed the H NMR of ((4-hexylphenyl)(10H-phenoxazin-10-yl)methanone where the aromatic signals exhibited from 6.9 ppm to 7.35 ppm and the aliphatic signals exhibited from 0.8 ppm to 2.65 ppm. Next, the (3,7-dibromo-10H-phenoxazin-10-yl)(4-hexylphenyl)methanone (DBrHP-POzM) monomer was synthesized via a bromination reaction of the HP-POzM monomer. Figure 2 shows the H NMR spectrum of DBrHP-POzM, which showed all characteristic peaks of the monomer. The peak appearing at 2.61 ppm was attributed to the methylene protons of the side chain. The peaks from 6.85 ppm to 7.37 ppm which are corresponding to the aromatic signals of monomers while the peaks from 0.8 ppm to 2.6 ppm that are attributed to the aliphatic signals of monomer.

The 1H NMR spectrum of ((4-hexylphenyl)(10H-phenoxazin-10-yl)methanone.

1H NMR spectrum of the (3,7-dibromo-10H-phenoxazin-10-yl)(4-hexylphenyl)methanone (DBrHP-POzM) monomer.
The polymer (4-hexylphenyl)(3-methyl-7-(7-methyl-9,9-dioctyl-9H-fluoren-2-yl)-10H-phenoxazin-10-yl)methanone (PO1) was synthesized via Suzuki-Miyaura polycondensation, which was carried out in toluene for 72 hours at 100 °C. Polymer P01 was prepared by reaction between (3,7-dibromo-10H-phenoxazin-10-yl)(4-hexylphenyl)methanone with -(9,9-dioctyl-9H-fluorene-2,7-diyl)bis[4,4,5,5-tetramethyl-1,3,2-dioxaborolan (128.5 mg, 0.2 mmol) monomer.

Synthesis of polymer (4-hexylphenyl)(3-methyl-7-(7-methyl-9,9-dioctyl-9H-fluoren-2-yl)-10H-phenoxazin-10-yl)methanone (PO1) via Suzuki-Miyaura polycondensation.
At the early stage of the reaction, the color of the mixture was light yellow, changed to green after 2 h and turned dark green after 12 h. After the reaction finished, polymer P01 was dissolved in CHCl and filtrated via a celite layer to eliminate the Pd catalyst, and then the polymers were obtained by precipitation in cold methanol. Then, the polymer was collected and characterized via GPC analysis. The GPC result showed that the average molecular weight of PO1 was 4500 g/mol with dispersity index of 1.65. This result suggested that the polymerization has been performed successfully via Suzuki-Miyaura cross coupling.

GPC trace of conjugated polymer PO1 in THF.
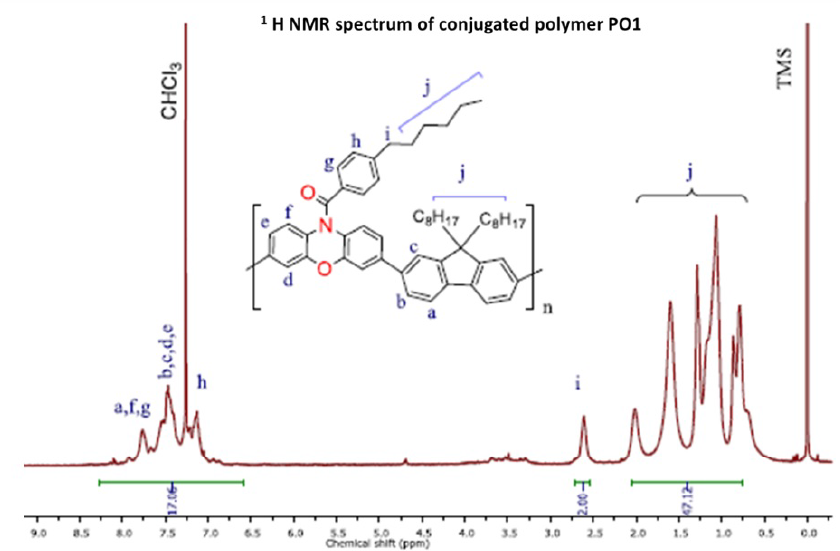
The structure of PO1 was characterized by FTIR and H NMR spectroscopies with TMS as a standard on a Bruker Avance 500 MHz. Figure 5 presents the FTIR spectrum of the polymer film measured by the ATR technique. Figure 4 shows the H NMR spectrum of the conjugated polymer, which was analyzed in CDCl. In H NMR spectrum, the signals appeared from 7.0 ppm to 8.0 ppm which is corresponding to the aromatic protons of PO1, whereas the signals presented from 0.8 ppm to 2.6 ppm which assigned to protons in side chain of PO1.

The FTIR spectrum of conjugated polymer PO1.

1H NMR spectrum of conjugated polymer PO1.
The absorption properties of the conjugated polymer PO1 were investigated by UV‒Vis spectroscopy in different solvents, including chloroform, tetrahydrofuran, toluene and a solid-state film. Figure 7 and Figure 8 show the UV‒Vis spectrum of PO1 and PL spectroscopy, respectively.

UV‒Vis spectrum of conjugated polymer PO1 in solution and in a solid-state film.

PL spectra of the conjugated polymer PO1 in chloroform, toluene and tetrahydrofuran.
Discussion
Figure 1 shows the H NMR spectrum of ((4-hexylphenyl)(10H-phenoxazin-10-yl)methanone, which showed all characteristic peaks of the compound. The peaks from 6.80 ppm to 7.45 ppm correspond to the protons in the aromatic monomer, and the peak at 2.6 ppm is assigned to methylene protons in hexyl side chains. The peaks from 0.8 ppm to 1.61 ppm are attributed to aliphatic side chains. The peaks from 0.8 ppm to 1.61 ppm are attributed to aliphatic side chains. The peak appearing at 2.61 ppm was attributed to the methylene protons of the side chain. The peaks from 6.80 ppm to 7.45 ppm are assigned to aromatic protons, and the peaks from 0.80 ppm to 1.70 ppm correspond to the aliphatic protons in the side chain. Based on the H NMR results, it is clear that the monomer DBrHP-POzM was synthesized successfully via 2 steps, including a C-N coupling reaction and bromination.
In the polymerization, the yield was calculated to be approximately 29%. The polymer PO1 exhibited good solubility in common solvents, such as chloroform, THF, dichloromethane and toluene. The PO1 polymers were characterized via gel permeation chromatography (GPC) to determine the relative molecular weights of the polymers. PO1 exhibited an average molecular weight of 4.500 g/mol with a polydispersity index (Đ) of 1.65. Figure 1 shows the GPC traces of PO1 in THF that show the mono distribution curves.
The FTIR spectrum of PO1 displayed bands between 2850 and 3062 cm due to C=C stretching of the aromatic structure and C-H deformation vibrations. The peaks at 1585 cm and 1491/1473 cm are ascribed to aromatic C=C stretching and aromatic C-H deformation vibrations, respectively. In addition, the peaks at 1668 cm are ascribed to the C=O stretching of 4-hexylphenyl)methanone units. Figure 1 and Figure 2 present the FTIR and H NMR spectra of the conjugated polymer, respectively.
In H NMR spectroscopy, the peaks are from 6.90 ppm to 7.92 ppm, which correspond to the aromatic protons in the conjugated polymer structure. The peak appeared at 2.6 ppm, which is attributed to methylene protons of hexyl side chains. In addition, the peaks from 0.8 ppm to 2.0 ppm are assigned to the protons of aliphatic side chains of monomer moieties. Thus, the H NMR spectrum of conjugated polymers showed all characteristic peaks. Based on these characterization results, Suzuki-Miyaura polycondensation was successfully performed to obtain the conjugated polymer PO1.
In solution, the conjugated polymer PO1 exhibited maximum absorption peaks at 405 nm, 410 nm and 415 nm in chloroform, toluene and tetrahydrofuran, respectively (Figure 7A). In the solid film, the conjugated polymer PO1 exhibited the maximum absorption peak at 400 nm for the nonannealed film and 420 nm for the annealed film. The onset absorption of conjugated polymer PO1 was determined at 600 nm, which corresponds to the optical bandgap of the polymer of approximately 2.09 eV. It should be noted that the band gap of PO1 is close to the band gap of the well-known poly(3-hexylthiophene) (P3HT).21 The maximum absorption peak of the annealed film of PO1 is higher than that of the nonannealed film. These results suggested that the annealing process at 150 °C caused the self-aggregation of conjugated polymer chains, which enhanced the absorption of conjugated polymers.22
Finally, the conjugated polymer PO1 in solution was investigated by the photoluminescence (PL) spectra in Figure 8 excited at 400 nm. In all solutions, the conjugated polymer PO1 displayed a doublet emission peak, including emission peaks at 470 nm and 563 nm. The fluorescence quantum yields (ФF) of these conjugated polymers in dilute CHCl were analyzed in comparison to 9,10-diphenylanthracene as a standard (ФF = 0.9). The ФF of PO1 has a value of 0.63, indicating a strong π–π stacking effect in polymer structures.23 This result indicated that the conjugated polymer may be a candidate material for optical sensory materials such as organic light emitting diodes or chemical sensor materials.
Conclusion
In conclusion, we have demonstrated that the novel conjugated polymer PO1 has been synthesized successfully based on ((4-hexylphenyl)(10H-phenoxazin-10-yl)methanone and 2,2'-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis[4,4,5,5-tetramethyl-1,3,2-dioxaborolan via Suzuki-Miyaura polycondensation. The conjugated polymers containing the fluorescent units exhibited good solubility in common solvents, which led to the processability of the thin film component in the electronic devices. The polymers also exhibited reasonable band gap energy levels of 2.09 eV, which are ponential materials for organic solar cell applications.
Acknowledgment(s)
This research was fully supported by the MURATA project under grant number “21VH04” and analysis instruments from DKSH Vietnam
Abbreviations
Proton nuclear magnetic resonance (1H NMR), Fourier transform infrared (FT-IR) spectroscopies, gel permeation chromatography (GPC), deuterated chloroform (CDCl¬3), (4-hexylphenyl)(10H-phenoxazin-10-yl)methanone (HP-POzM), (3,7-dibromo-10H-phenoxazin-10-yl)(4-hexylphenyl)methanone (DBrHP-POzM), (4-hexylphenyl)(3-methyl-7-(7-methyl-9,9-dioctyl-9H-fluoren-2-yl)-10H-phenoxazin-10-yl)methanone (PO1).
Author’s contribution
Thao Thanh Bui and Ha Tran Nguyen conceptualized the project methodology, wrote the original draft and supervised the investigation. Dat Hung Tran, Hai Minh Phan, Dai Ngoc Dao, Quoc-Thiet Nguyen, Son Thanh Cu analysed experimental data. Ha Tran Nguyen supervised the investigation and wrote the manuscript. All authors discussed and edited the manuscript.
Conflict of interest
The authors declare that they have no competing interest.

