One triterpenoid and five phenolic compounds from the liverwort Bazzania sp.
- Department of Organic Chemistry, University of Science, National UniversityHo Chi Minh City, Vietnam
- Department of Plant Biotechnology and Biotransformation, University of Science, National UniversityHo Chi Minh City, Vietnam
- Department of Chemistry, Ho Chi Minh City University of Education, Ho Chi Minh City, Vietnam
Abstract
Introduction: The Bazzania genus, a type of liverwort known as a low-level herbaceous organism, comprises approximately 250 species. Only 8 species have been chemically and biologically studied worldwide, and none have been studied in Vietnam. This paper described the isolation and structure elucidation of six compounds isolated from the liverwort Bazzania sp. collected in Lac Duong district, Lam Dong Province, Vietnam, in July 2020.
Methods: Phytochemical investigations of the n-hexane and chloroform extracts of Bazzania sp. led to the isolation of six pure compounds. Their chemical structures were elucidated by HR-ESI-MS, 1D and 2D-NMR spectroscopic analysis and comparison with previously published data.
Results: From the studied liverwort Bazzania sp., six compounds, namely, cycloartenol (1) and five phenolic derivatives, p-coumaric acid (2), p-hydroxybenzoic acid (3), methyl 2,4-dihydroxy-3,6-dimethylbenzoate (4), barbatic acid (5), and methyl 2-hydroxy-4-methoxy-6-tetradecylbenzoate (6), were isolated and structurally elucidated.
Conclusions: Although these compounds are known in other species, this is the first time they have been reported in Bazzania sp.
INTRODUCTION
Liverworts, the species of the division Marchantiophita, belong to Bryophytes, which are known as nonvascular plants. They are mostly spread in high moisture areas, humid tropical forests, and the banks of rivers and streams. 1 The genus, liverwort, comprises approximately 250 species.2, 3 In the world, only a few of them, 8 species, have been chemically and biologically studied, and none have been studied in Vietnam. 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Previous chemical studies on the eight mentioned species showed that they contained sesquiterpenes, diterpenes, and lignans. 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Some possess cytotoxic14 and nitric oxide inhibitory activity. 17 Herein, we report the investigation of the n-hexane and chloroform extracts of . collected in Lac Duong district, Lam Dong province, Vietnam. We isolated six compounds, including a triterpenoid compound, cycloartenol (1), and five phenolic derivatives, p-coumaric acid (2), p-hydroxybenzoic acid (3), methyl 2,4-dihydroxy-3,6-dimethylbenzoate (4), barbatic acid (5), and methyl 2-hydroxy-4-methoxy-6-tetradecylbenzoate (6). Six compounds were isolated via silica gel chromatographic processes, and their chemical structures were elucidated by HR-ESI-MS, 1D, and 2D NMR spectroscopic methods.
MATERIALS AND METHODS
General experimental procedures
The HR–ESI–MS was recorded on an MicrOTOF–Q mass spectrometer. H-NMR (600 MHz) and C-NMR (150 MHz) spectra were measured on a Bruker Avance 600 spectrometer using CDCl ( 7.26 and 77.16); (CD)CO ( 2.05 and 206.26, 29.84) and CDOD ( 4.87 and 49.0) were used both as solvents and internal references.
Thin layer chromatography (TLC) was carried out on precoated silica gel 60 F (Merck), and the isolated compounds were visualized by spraying with vanillin + HSO in ethanol or methanol followed by heating. Gravity column chromatography was performed on silica gel 60 (0.040–0.063 mm, Himedia). Organic solvents were distilled prior to use.
Plant material
The liverwort . was collected on Lang Biang Mountain, Lac Duong District, Da Lat city, Lam Dong Province, at an altitude of 150 meters in July 2020. The sample of . was authenticated by MSc. Luong Thien Tam, Department of Ecology and Evolution Biology. University of Science, National University–Ho Chi Minh City. A voucher specimen, coded BazlB, was deposited in the herbarium of the Laboratory of Plant Biotechnology, Department of Plant Biotechnology and Biotransformation, Faculty of Biology and Biotechnology.
Extraction and isolation
The ground powder sample (0.37 kg) of was extracted with methanol by maceration at room temperature to obtain a crude methanolic residue (29.59 g), which was subjected to silica gel solid-phase extraction and eluted consecutively with -hexane, chloroform, ethyl acetate, and methanol at ambient temperature. Then, each type of eluent was evaporated at low pressure to afford -hexane (3.97 g), chloroform (6.43 g), ethyl acetate (6.82 g), and methanol (10.24 g) residues.
The -hexane residue was chromatographed over silica gel with -hexane: CHCl (10:0) to (0:10) to give 10 fractions, coded HE1 (283 mg), HE2 (560 mg), HE3 (495 mg), HE4 (233 mg), HE5 (170 mg), HE6 (400 mg), HE7 (590 mg), HE8 (201 mg), HE9 (487 mg), and HE10 (228 mg). The residue of fraction HE7 was further chromatographed (CC) over silica gel with -hexane:chloroform (100:0, 90:10, 0:100) to give five subfractions (HE7.1-HE7.5). The subfraction HE7.2 (192 mg) was silica gel chromatographed with a gradient system of -hexane:toluene (90:10, 80:20, 70:30, 10:90) to afford compound 6 (6 mg). Subfraction HE7.5 (152 mg) was subjected to CC using the mobile phase -hexane:chloroform (90:10, 85:15, 50:50, 10:90) to afford compound 1 (5 mg).
The chloroform residue was silica gel chromatographed and eluted by -hexane:chloroform (80:20, 50:50, 0:100) and chloroform:ethyl acetate (90:10, 80:20, 0:100) to give seven fractions (CL1‒CL7). The fraction CL4 (840 mg) was further fractionated by silica gel chromatography using -hexane:ethyl acetate (stepwise, 98:2, 95:5, 90:10, 0:100) to obtain six subfractions (CL4.1‒CL4.6). Further fractionation of CL4.2 (187 mg) and elution with-hexane:ethyl acetate (90:10 to 0:100) afforded compound 4 (7 mg). The same procedure was applied to subfraction CL4.6 (200 mg), eluted by -hexane:ethyl acetate (90:10 to 0:100) to obtain compound 5 (4 mg). Fraction CL6 (540 mg) was divided into four subfractions (CL6.1‒CL6.4) by CC using -hexane:acetone (99:1, 95:5, 90:10, 80:20, 0:100). Subfraction CL6.4 (213 mg) was repeatedly chromatographed followed by preparative TLC to afford compound 2 (3 mg) and compound 3 (4 mg).
RESULTS
Using silica gel column chromatography, the chemical investigation of the -hexane and chloroform extracts of the liverwort sp. led to the isolation of six compounds. Their structures are shown in Figure 1 by using 1D and 2D NMR and HR-ESI-MS.

Chemical structures of six compounds isolated from the liverwort Bazzania sp.
DISCUSSION
The chemical structures of six isolated compounds were elucidated based on modern spectroscopic methods such as HR-ESI-MS and 1D and 2D NMR, and their data were compared with those in the literature. Compounds possessing a similar framework were structurally discussed.
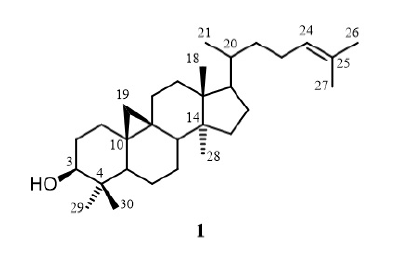
Compound 1 (5 mg) was obtained as a white solid that was highly soluble in chloroform. The HR-ESI-MS spectrum (positive mode) showed a pseudomolecular ion peak at 427.4030 [M+H] (calcd. for CHO+H, 427.3934). The H-NMR spectrum showed signals of seven methyl groups [ 0.81 (3H, , H-29), 0.96 (6H, , H-18, H-28), 0.91 (3H, 6.5 Hz, H-21), 0.93 (3H, , H-30), 0.96 (3H, , H-18), 1.60 (3H, , H-27), 1.66 (3H, , H-26)], two nonequivalent methylene protons [ 0.37 (1H, , 3.5 Hz, H-19a) and 0.58 (1H, , 3.5 Hz, H-19b)], one oxymethine [ 3.22 (1H, , 10.0, 5.5 Hz, H-3)] and one olefinic proton [ 5.12 (1H, , H-24)]. The C-NMR spectrum showed thirty signals with two olefinic carbons [ 125.98 (C-24) and 131.25 (C-25)], one oxygenated methine carbon [ 78.45 (C-3)], and many signals in the high-field zone (14‒53 ppm). The good compatibility of its NMR and MS data with those in the literature19, 20proposed that 1 was cycloartenol.
Compound 2 (3 mg) was obtained as colorless needles and was highly soluble in methanol. The NMR data (
Compound 3 (4 mg) was isolated as a white solid that was highly soluble in MeOH. Its HR-MS spectrum showed a deprotonated molecular ion peak at 137.0103 [M–H] (calcd. for CHO‒H, 137.0244), as well as good compatibility in the comparison of its NMR data with those in the literature22,suggested that 3 was -hydroxybenzoic acid.
Compound 4 (7 mg) was isolated as a white amorphous powder. Its molecular formula was determined to be CHO through its pseudomolecular ion peak at 195.0673 [M–H] (calcd. for CHO‒H, 195.0662) in the HR-ESI-MS spectrum. The H-NMR spectrum showed one aromatic methine proton at 6.21 (3H, ), two methyl groups at 2.10 (3H, ) and 2.46 (3H, ), and one methoxy group at 3.92 (3H, ). The C-NMR spectrum showed 10 resonances of one benzene ring, two methyl groups, and one methoxycarbonyl group (

Selected HMBC correlations of compounds 4, 5, and 6
Compound 5 (4 mg) was isolated as a white solid that was highly soluble in acetone. The H-NMR spectrum showed four methyl signals [1.99 (3H, , H-8′), 2.03 (3H, , H-8), 2.61 (3H, , H-9′), 2.69 (3H, , H-9)], one methoxy group [ 3.93 (1H, , 4-OCH)] and two aromatic protons of two benzene rings at 6.43 (1H, , H-5′) and 6.59 (1H, , H-5). The spectrum also showed one chelated hydroxyl group at 11.59 (1H, , 2-OH). The C-NMR spectrum (
Compound 6 (6 mg) was isolated as a white amorphous solid that was highly soluble in chloroform. Its NMR data (
The 1H-NMR data of compounds 1 and 6
|
No. |
Compound 1 (Acetone-d6) |
Compound 6 (CDCl3) | ||
|
δH (J in Hz) |
δC |
δH (J in Hz) |
δC | |
|
1 |
1.25 (1H; m) 1.56 (1H; m) |
32.8 |
104.3 | |
|
2 |
1.68 (1H; m) 1.60 (1H; m) |
31.3 |
164.1 | |
|
3 |
3.22 (1H; dd; 10.5; 5.0 Hz) |
78.5 |
6.33 (1H; d, 2.7) |
98.9 |
|
4 |
– |
41.3 |
165.7 | |
|
5 |
1.31 (1H; m) |
48.2 |
6.28 (1H; d, 2.7) |
110.8 |
|
6 |
1.60 (1H; m) 0.83 (1H; m) |
21.9 |
148.3 | |
|
7 |
1.09 (1H; m) 1.34 (1H; m) |
26.1 |
170.3 | |
|
8 |
1.56 (1H; m) |
49.0 | ||
|
9 |
– |
20.6 | ||
|
10 |
– |
26.9 |
2.84 (2H; t, 6.5) |
37.1 |
|
11 |
2.03 (1H; m) 1.18 (1H; m) |
27.1 |
1.52 (2H) |
32.1 |
|
12 |
1.66 (2H; m) |
33.8 |
1.29 (2H) |
30.0 |
|
13 |
– |
46.1 |
1.29 (2H) |
30.0 |
|
14 |
– |
49.6 |
1.29 (2H) |
30.0 |
|
15 |
1.33 (2H; m) |
36.3 |
1.29 (2H) |
30.0 |
|
16 |
1.33 (1H; m) 1.93 (1H; m) |
28.8 |
1.29 (2H) |
30.0 |
|
17 |
1.64 (1H; m) |
53.1 |
1.29 (2H) |
30.0 |
|
18 |
0,94 (3H; s) |
18.6 |
1.29 (2H) |
30.0 |
|
19 |
0.37 (1H; d; 3.5 Hz; H-19a) 0.58 (1H; d; 3.5 Hz; H-19b) |
30.5 |
1.29 (2H) |
30.0 |
|
20 |
1.43 (1H; m) |
36.7 |
1.29 (2H) |
30.0 |
|
21 |
0.91 (3H; d; 6.5 Hz) |
18.7 |
1.29 (2H) |
30.0 |
|
22 |
1.07 (1H; m) 1.48 (1H; m) |
37.1 |
1.25 (2H) |
22.8 |
|
23 |
1.90 (1H; m) 2.06 (1H; m) |
25.5 |
0.89 (3H; t; 6.6) |
14.2 |
|
24 |
5.12 (1H; m) |
125.9 |
11.69 |
55.4 |
|
25 |
– |
131.2 |
3.80 (3H; s) |
52.0 |
|
26 |
1.60 (3H; s) |
17.7 |
3.92 (3H; s) | |
|
27 |
1.66 (3H; s) |
25.9 | ||
|
28 |
0.93 (3H; s) |
19.8 | ||
|
29 |
0.96 (3H; s) |
25.6 | ||
|
30 |
0.81 (3H; s) |
14.7 | ||
1H-NMR data of compounds 2-5
|
No. |
2 (Methanol-d4) |
3 (Methanol-d4) |
4 (CDCl3) |
5 (Acetone-d6) |
|
1 | ||||
|
2 |
7.43 (1H; d 8.6) |
7.87 (1H; d 8.6) | ||
|
3 |
6.84 (1H; d 8.6) |
6.79 (1H; d 8.7) | ||
|
4 | ||||
|
5 |
6.84 (1H; d 8.6) |
6.79 (1H; d 8.7) |
6.21 (1H; s) |
6.59 (1H; s) |
|
6 |
7.43 (1H; d 8.6) |
7.87 (1H; d 8.6) | ||
|
7 |
7.63 (1H; d 15.6) | |||
|
8 |
6.25 (1H; d 15.6) |
2.10 (3H; s) |
2.03 (3H; s) | |
|
9 |
2.46 (3H; s) |
2.69 (3H; s) | ||
|
5′ |
6.43 (1H; s) | |||
|
8′ |
1.99 (3H; s) | |||
|
9′ |
2.61 (3H; s) | |||
|
2-OH |
12.00 (1H; s) |
11.59 (1H; s) | ||
|
4-OH |
5.07 (1H; s) | |||
|
4-OCH3 |
3.93 (3H; s) | |||
|
7-OCH3 |
3.92 (3H; s) |
13C-NMR data of compounds 1-6
|
No. |
2 (CD3OD) |
3 (CD3OD) |
4 (CDCl3) |
5 (Acetone-d6) |
|
1 |
115.1 |
125.3 |
105.5 |
105.4 |
|
2 |
116.1 |
132.8 |
163.3 |
163.2 |
|
3 |
130.3 |
115.8 |
108.7 |
111.1 |
|
4 |
159.7 |
162.4 |
158.2 |
162.9 |
|
5 |
130.3 |
115.8 |
110.7 |
107.3 |
|
6 |
116.1 |
132.8 |
140.3 |
141.6 |
|
7 |
145.8 |
172.5 |
172.7 |
171.1 |
|
8 |
126.6 |
7.8 |
7.8 | |
|
9 |
170.4 |
24.2 |
24.8 | |
|
1′ |
111.1 | |||
|
2′ |
162.9 | |||
|
3′ |
115.5 | |||
|
4′ |
151.3 | |||
|
5′ |
114.8 | |||
|
6′ |
141.6 | |||
|
7′ |
177.2 | |||
|
8′ |
9.4 | |||
|
9′ |
23.5 | |||
|
10′ | ||||
|
11′ | ||||
|
12′ | ||||
|
13′ | ||||
|
14′ | ||||
|
4-OCH3 |
55.1 | |||
|
7-OCH3 |
51.8 |
CONCLUSION
Six compounds were isolated from the -hexane and chloroform extracts of the liverwort , collected at Lac Duong district, Lam Dong province, Vietnam, using chromatographic methods. Those compounds are cycloartenol (1) and five phenolic derivatives, -coumaric acid (2), -hydroxybenzoic acid (3), methyl 2,4-dihydroxy-3,6-dimethyl benzoate (4), barbatic acid (5), and methyl 2-hydroxy-4-methoxy-6-tetradecyl benzoate (6). Although 1-5 were known in other species, this is the first time they have been reported in . After being checked in Scifinder (on September 30, 2022), compound 6 was determined to be new. Further research on the remaining extracts is ongoing.
ABBREVIATIONS
HR-ESI-MS: High resolution- Electrospray ionization-Mass spectrometry
H-NMR: Proton nuclear magnetic resonance
C-NMR: Carbon-13 Nuclear Magnetic Resonance
HMBC: Heteronuclear Multiple Bond Correlation
: singlet
: doublet
: doublet of doublets
: triplet
: multiplet
COMPETING INTEREST
The authors declare no competing financial interest.
AUTHORS’ CONTRIBUTION
Tran Q. T. collected samples and prepared extracts. Nguyen N. N., Nguyen T. B. T., and Nguyen H. C. performed column chromatography on the -hexane extract and isolated compounds. Nguyen X. K., Phan T. T., and Le H. K. performed column chromatography on the chloroform extract and isolated compounds. Duong T. H and Huynh N. V. interpreted the NMR and MS data. Nguyen X. K. prepared the manuscript and searched the bibliography. Nguyen K. P. P. gave the final correction for the manuscript.

