Comparison of the total phenolic content and antioxidant and antibacterial activities of different fractions obtained from selected plant leaves native to Viet Nam
- Faculty of Environmental and Food Engineering, Nguyen Tat Thanh University, Ho Chi Minh City 754000, Viet Nam
Abstract
In this study, five types of plant extracts in Vietnam were selected to compare phenolic content, antioxidant and antibacterial activities, respectively Gymnanthemum amygdalinum (bitter leaf), Piper betle (betel), Pseuderanthemum bracteatum (Imlay), Piper sarmentosum (kaduk), and Paederia tomentosa (stinkvine). Five types of leaves were fractionally extracted with n-hexane (HE), ethyl acetate (EA) and water (W) solvents. The antioxidant activity was compared based on free radical scavenging capacity (DPPH, ABTS), iron reducing capacity (FRAP) and iron chelation capacity (FIC). At the same time, the total phenolic content (TPC) was also compared by the Folin-Ciocalteau method. The results demonstrate antioxidant activity (DPPH), the EA fraction of betel leaf was the best, followed by four extracts of bitter leaf > kaduk > Imlay > stinkvine. Similarly, the EA fraction of betel leaf also showed the highest FRAP and ABTS iron-reducing activity. A correlation between phenolic content and antioxidant activities of leaf extracts was also investigated. Regarding antibacterial activity, betel leaf in all fractions showed the highest antibacterial activity against most gram (+) and gram (-) organisms through diffuse on agar plate test. Meanwhile, bitter leaf showed the lowest antibacterial activity in both EA and W fractions.
Introduction
Natural plant sources are rich in vitamins, minerals and phytochemicals, such as phenols and flavonoids, which exhibit good antioxidant activity and can also chelate metal ions 1. The antioxidant mechanism of phytochemical compounds relies on scavenging free radicals to help strengthen cell defenses, thereby indirectly reducing the potential for tissue damage. In addition, carotenoids, tocopherols, ascorbates and phenolics are correlated with a reduced risk of cancer, cardiovascular disease, neurodegenerative disease, and inflammation 2, 3. Currently, several studies are being carried out on bioactive compounds such as phenolics and flavonoids due to their many health benefits to humans through their antioxidant capacity4, 5.
Many plant species have been used as food and pharmaceutical sources because of their nutritional and pharmacological properties 6. Most modern medicines are derived from ancient herbs and have been used for centuries as human remedies because of their antifungal, antibacterial and antiprotozoal activities7. In recent years, an increasing number of antibacterial properties of medicinal plants have been reported from different regions of the world 8 since the utilization of plant-derived secondary metabolites may be another approach to overcome the escalating problems of drug-resistant infections 9. Consequently, natural antioxidant molecules are currently the subject of research on their life applications.
L., also called bitter leaf, belongs to the Asteraceae family and is found in Asia and Africa (mainly in western African countries), with approximately 300 species in Mexico and southern and central America 10. leaves contain many phytochemicals, such as tannins, saponins, triterpenoids, polyphenols, flavonoids, and amino acids, which enhance their pharmacological properties 10, 11, 12. Extraction of leaves in methanol and chloroform inhibited the pathogenic bacteria , , and and two fungal species ( and13.
L. (betel) is a climbing plant belonging to the family Piperaceae. It is commonly grown in Asian countries, such as Sri Lanka, India, Malaysia and Thailand14. In addition, Betel leaves contain high amounts of essential oils, mainly cadinene, carvacrol, allyl catechol, chavicol, p-cymene, caryophyllene, chavibetol, cineole and estragol13, 15. This plant has been shown to possess medicinal properties, including gastro-protective, wound healing and hepato-protective effects, ascribed mainly to bioactive phenolic compounds 16. Furthermore, betel leaf extract has been shown to reduce and inhibit lipid peroxidation and enhance the levels of natural antioxidants, such as vitamins C and E17.
(Imlay) belongs to the family Acanthaceae and is a common plant species in Vietnam18. The roots of these plants contain several highly bioactive compounds, such as lupeol, lupenone, betulin and pomolic acid; in particular, lupeol and betulin have antibacterial, antioxidant and cytotoxic effects on liver and breast cancer cells 19. In addition, the study of Dechayont . 20 showed that phenolics found in Imlay fruits have high antioxidant activity. (Lour.) Merr. (stinkvine) is commonly grown in China, Bangladesh, India and Mauritius. In recent years, stinkvine has been reported to have anticancer, anticonvulsant, hepatoprotective and anti-inflammatory activities21, 22, 23, 24. (kaduk) belongs to the family Piperaceae and is found in hot and humid climates. Kaduk is widely grown in the southeastern coastal areas of China and Southeast Asian countries25. The study on biological activities of kaduk extract showed Kaduk has antioxidant 26, 27, anti-inflammatory and antipyretic, neuromuscular blocking28, killing larvae29, inhibition of α-glucosidase30, proliferation of lymphocytes 31, hypoglycemia 32, resistance to allergens33.
Although these plants have many antioxidant and antibacterial properties, research on plants grown in Vietnam is still limited. This study aimed to compare the antioxidant and antibacterial properties of three solvent fractions, namely, n-hexane (HE), ethyl acetate (EA) and water (W), obtained from the fractionation of five leaves.
Description of plant leaves used in this study
|
No. |
Botanical name |
Common name |
Family |
Geographical origin |
|---|---|---|---|---|
|
1 |
Gymnanthemum amygdalinum |
Bitter leaf |
Asteraceae |
Di Linh, Lam Dong province |
|
2 |
Piper betle |
Betel |
Piperaceae |
Dak To, Kon Tum province |
|
3 |
Pseuderanthemum bracteatum |
Imlay |
Acanthaceae |
Di Linh, Lam Dong province |
|
4 |
Piper sarmentosum |
Kaduk |
Piperaceae |
Dak To, Kon Tum province |
|
5 |
Paederia tomentosa |
Stinkvine |
Rubiaceae |
Dak To, Kon Tum province |
Comparison of total phenolic content (TPC, mg GAE/L), ferric reducing antioxidant power (FRAP, g TE/L), DPPH free radical scavenging activity (mg TE/L), and ABTS cation radical scavenging activity (mg TE/L) of different fractions obtained from five plant leaves
|
Fraction* |
Dried weight (g) |
TPC |
FRAP |
DPPH |
ABTS |
|---|---|---|---|---|---|
|
Bitter leaf | |||||
|
HE |
0.21 |
306.06 (4.79) |
529.23 (10.29) |
57.48 (0.97) |
175.19 (1.82) |
|
EA |
0.58 |
113.54 (1.52) |
211.97 (5.93) |
648.74 (16.53) |
1167.99 (16.04) |
|
W |
2.96 |
729.76 (4.21) |
1184.19 (2.19) |
3419.94 (55.7) |
3368.97 (97.70) |
|
Total |
1149.36 |
1925.39 |
4126.15 |
4712.16 | |
|
Betel | |||||
|
HE |
0.20 |
500.90 (4.54) |
1021.06 (7.48) |
2270.81 (29.10) |
2198.13 (11.14) |
|
EA |
0.77 |
815.99 (5.96) |
1384.40 (9.46) |
21225.38 (392.20) |
27630.03 (825.94) |
|
W |
3.63 |
778.20 (1.85) |
1183.42 (2.85) |
1509.17 (31.55) |
332.50 (4.24) |
|
Total |
2095.09 |
3588.88 |
25005.35 |
30160.66 | |
|
Imlay | |||||
|
HE |
0.12 |
27.87 (0.44) |
136.80 (1.37) |
416.84 (11.88) |
69.94 (1.98) |
|
EA |
0.29 |
199.83 (2.76) |
328.26 (9.02) |
641.91 (5.93) |
414.13 (9.99) |
|
W |
2.63 |
487.30 (9.98) |
649.20 (9.4) |
814.54 (21.08) |
1437.27 (38.81) |
|
Total |
715.00 |
1114.26 |
1873.30 |
1921.35 | |
|
Kaduk | |||||
|
HE |
0.35 |
771.80 (5.20) |
1191.48 (3.31) |
82.46 (0.21) |
197.50 (5.12) |
|
EA |
0.52 |
639.39 (4.91) |
901.30 (22.83) |
214.82 (3.63) |
299.59 (2.81) |
|
W |
4.51 |
28.21 (0.21) |
17.69 (0.42) |
2053.97 (58.43) |
2716.68 (26.06) |
|
Total |
1439.40 |
2110.46 |
2351.26 |
3213.77 | |
|
Stinkvine | |||||
|
HE |
0.36 |
53.13 (0.99) |
90.81 (2.51) |
38.01 (0.58) |
168.24 (1.67) |
|
EA |
0.36 |
481.77 (6.80) |
419.89 (5.06) |
148.34 (4.22) |
473.14 (14.02) |
|
W |
2.61 |
767.09 (5.04) |
1087.50 (27.19) |
635.51 (16.60) |
1459.12 (21.81) |
|
Total |
1301.99 |
1598.21 |
821.85 |
2100.50 |
Pearson correlation between the contents of phenolics (TPC), and antioxidant activities (DPPH free radical scavenging activity, ABTS cation radical scavenging activity, ferric reducing antioxidant power – FRAP) of different fractions obtained from five plant leaves
|
TPC |
FRAP |
DPPH |
ABTS | |
|---|---|---|---|---|
|
TPC |
1 | |||
|
FRAP |
0.963** |
1 | ||
|
DPPH |
0.369 |
0.456 |
1 | |
|
ABTS |
0.351 |
0.428 |
0.996** |
1 |
Antibacterial activity of leaf fractions against eleven pathogens as presented in diameter of inhibition zones using agar well diffusion assay
|
Inhibition zone (mm) | |||||||||||
|
Shi |
Esc |
Cit |
Sal |
Vib |
Pro |
Cam |
Sta |
Bac |
Lis |
Can | |
|
Bitter leaf | |||||||||||
|
HE |
n.d. |
17 |
n.d. |
n.d. |
13 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
|
EA |
26 |
17 |
22 |
20 |
30 |
26 |
17 |
25 |
21 |
17 |
26 |
|
W |
n.d. |
16 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
|
Betel | |||||||||||
|
HE |
16 |
14 |
14 |
15 |
16 |
13 |
15 |
16 |
0 |
14 |
15 |
|
EA |
25 |
18 |
20 |
22 |
20 |
18 |
2 |
26 |
17 |
21 |
26 |
|
W |
n.d. |
12 |
12 |
10 |
14 |
11 |
11 |
9 |
n.d. |
11 |
n.d. |
|
Imlay | |||||||||||
|
HE |
n.d. |
13 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
13 |
n.d. |
n.d. |
n.d. |
|
EA |
18 |
18 |
23 |
20 |
33 |
22 |
21 |
33 |
n.d. |
15 |
22 |
|
W |
11 |
12 |
11 |
11 |
19 |
11 |
n.d. |
15 |
n.d. |
n.d. |
11 |
|
Kaduk | |||||||||||
|
HE |
n.d. |
12 |
n.d. |
n.d. |
14 |
n.d. |
n.d. |
14 |
n.d. |
n.d. |
n.d. |
|
EA |
16 |
15 |
15 |
14 |
17 |
14 |
16 |
20 |
n.d. |
13 |
14 |
|
W |
15 |
12 |
13 |
13 |
14 |
n.d. |
9 |
17 |
n.d. |
13 |
15 |
|
Stinkvine | |||||||||||
|
HE |
n.d. |
n.d. |
n.d. |
n.d. |
13 |
n.d. |
n.d. |
11 |
n.d. |
n.d. |
n.d. |
|
EA |
14 |
14 |
13 |
14 |
16 |
12 |
16 |
26 |
n.d. |
15 |
14 |
|
W |
10 |
9 |
10 |
9 |
8 |
10 |
11 |
11 |
n.d. |
12 |
11 |
|
Ref* |
40 |
28 |
26 |
28 |
27 |
37 |
39 |
28 |
24 |
38 |
35 |
Materials and methods
Materials, microorganisms, and chemicals
Five wild plants, namely, (bitter leaf), (betel), (Imlay), (kaduk), and (stinkvine), were studied, and their botanical names, common names, families, and geographical origins are presented in
Pathogenic microorganisms, including seven gram-negative bacteria ( ATCC 9290, ATCC 8739, ATCC 8090, ATCC 6539, ATCC 17802, ATCC 25933, ATCC 33291), three gram-positive bacteria ( ATCC 6538, ATCC 11778, ATCC 13932), and one yeast strain ( ATCC 10231), were kept frozen in Mueller–Hinton broth (MHB) medium containing 15% v/v glycerol.
Gallic acid, DPPH, TPTZ, ABTS, and Trolox were obtained from Sigma‒Aldrich (Singapore). Folin–Ciocalteu reagent (2 N) was prepared from solid sodium tungstate, sodium molybdate, and lithium sulfate. Ampicillin and Mueller–Hinton media were obtained from Hi-Media Laboratory (Mumbai, India).
Methanol, n-hexan, ethyl acetate, hydrochloric acid, potassium chloride, aluminum chloride monohydrate, sodium hydroxide, ferric chloride hexahydrate, ferrous sulfate, potassium dihydrogen phosphate, potassium ferricyanide, and other chemicals were of analytical grade.
Preparation of plant fractions
The dried leaf material (10 g) was macerated with 250 mL of 80% v/v methanol at room temperature for 3 days. After maceration, the mixture was filtered through Whatman No. 2 filter paper to remove insoluble components. The filtrate was acquired and evaporated under vacuum in a Hei-VAP Value rotary vacuum evaporator (Heidolph Instruments, Schwabach, Germany) at 55°C to remove solvent. The concentrate was then diluted to 100 mL with distilled water and fractionated with 50 mL of different solvents in order of increasing polarity, including n-hexane and ethyl acetate, using a separating funnel to obtain three fractions: the n-hexane fraction (HE), the ethyl acetate fraction (EA), and the residual aqueous fraction (W). These fractions were also dried to calculate the dry weight of each fraction.
Antioxidant activities
To prepare the analytical solutions for HE and EA, 1 mL of each fraction was transferred to a Petri dish where the solvent (n-hexane and ethyl acetate) had evaporated spontaneously. The residues were then redissolved and diluted to 10 mL using distilled water, while the W fractions were used directly as analytical solutions.
Total phenolic content (TPC)
The total phenolic content was determined according to the Folin–Ciocalteu method described in ISO 14502–1:2005 34 based on the reaction of antioxidants with Folin–Ciocalteu reagent in an alkaline medium to form a blue chromophore with maximum absorption at 765 nm. The phenolic content was calculated based on the gallic acid standard curve and is expressed in mg gallic acid equivalent per liter of extract (mg GAE/L).
DPPH free radical scavenging activity
Antioxidant activity was evaluated through DPPH free radical scavenging capacity based on the change in the purple color of the DPPH solution (0.6 mM) measured at 515 nm upon reaction with antioxidants 35. The antioxidant activity of DPPH was calculated against the Trolox calibration curve and expressed in mg Trolox equivalent per liter of extract (mg TE/L).
ABTS cation radical scavenging activity
ABTS free radical scavenging activity was determined based on the discoloration of ABTS (7.4 mM) solution measured at 734 nm upon reaction with the antioxidant 36. The ABTS cationic radical scavenging activity was calculated against the Trolox calibration curve and expressed in mg Trolox equivalent per liter of extract (mg TE/L).
Ferric reducing antioxidant power
Ferric reducing antioxidant power (FRAP) was determined according to37 based on the chromophores formed between the working reagents (a mixture of 0.3 M acetate buffer at pH 3.6, 0.01 M TPTZ prepared in 0.04 M HCl, and 0.02 M FeCl.6HO solution at a volumetric ratio of 10:1:1) and antioxidants. Ferric reducing antioxidant activity was calculated against the Trolox calibration curve and expressed in mg Trolox equivalent per liter of extract (mg TE/L).
Antibacterial activity – Agar well diffusion test
The antibacterial activities of the leaf fractions were determined by the agar well diffusion method as described in the literature38. The bacterial pathogens were grown in liquid media for 20 h for a final microorganism concentration of 10 CFU/mL. Subsequently, 100 mL of the test strains was spread over the surface of the agar disk. The sterilized filter paper discs were loaded with 50 mL of leaf fractions, and ampicillin (0.2 mg/mL) was used as a positive control before they were incubated at 37°C for 18 h. Finally, the inhibition zone diameter (mm), which represents the extent of bacterial inhibition of the extracts compared with that of the control samples, was measured.
Statistical analysis
All the statistical techniques, including the normality test, homoscedasticity of variances, one-way ANOVA, and post hoc Tukey test, were performed at the 5% significance level by using R version 4.1.2.
Results and discussion
Total phenolic content
Phenolic compounds are major antioxidant components that are involved in many biological and functional activities for human health 39, 40. The total phenolic contents of different fractions, such as n-hexane (HE), ethyl acetate (EA) and water (W), from bitter leaves, betel, Imlay, kaduk, and stinkvine are shown in

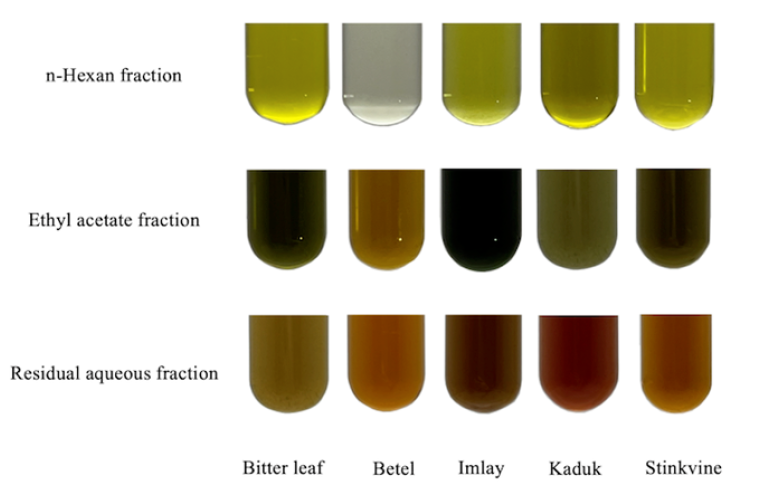
Visual appearance of different fractions obtained from five plant leaves.
The color of the extract of each leaf was different for each fraction, and the changes in color of the different fractions, such as n-hexane (HE), ethyl acetate (EA) and water (W), from bitter leaf, betel, Imlay, kaduk, and stinkvine are shown in Figure 1. In the HE fraction, the color of the extracts was mostly green with a yellowish tint. However, the betel leaf extract had a different gray color than the other leaf extracts because the color level increased or decreased depending on the leaf type and the solvent polarity. In the EA fraction, the color of the leaf extract that had begun to darken and turn black clearly changed; specifically, the Imlay leaf extract had the darkest black color. In the W fraction, the Imlay extract had the darkest brown color compared to the other leaf extracts. Differences in the color of leaf extracts from other fractions are due to differences in plant species, chlorophyll content and polarity of the solvent used43.
DPPH and ABTS free radical scavenging activities
DPPH is a free radical widely used for evaluating antioxidant potential through its free radical scavenging activity44. The DPPH free scavenging activities of different fractions, such as n-hexane (HE), ethyl acetate (EA) and water (W), from bitter leaves, betel, Imlay, kaduk, and stinkvine are shown in
In addition, the ABTS free radical scavenging method is a more sensitive and stable method used in media with different pH values and is often used to evaluate the antioxidant capacity of polyphenol compounds46.
FRAP
The FRAP free scavenging activities of different fractions, such as n-hexane (HE), ethyl acetate (EA) and water (W), from bitter leaves, betel, Imlay, kaduk, and stinkvine are shown in
In addition, the results also showed the variation in FRAP values among the different fractions. The FRAP values of three of the five leaf types (bitter leaf, Imlay, and stinkvine) were greater for the W fraction than for the other two fractions, ranging from 629.20 to 1184.19 g TE/L. In contrast, betel and kaduk extracted by EA (1384.40 mg TE/L) and HE (1191.48 g TE/L) solvents exhibited higher FRAP activity than did those extracted by W. Similar results were also reported in the studies of Guleria . 50 on the fractions of fruit and Park .51 on the fractions of cultivated with .
Correlation
Correlations between total phenolic content (TPC) and antioxidant capacities (FRAP, DPPH and ABTS free radical scavenging activity) of different fractions, such as n-hexane (HE), ethyl acetate (EA) and water (W), from bitter leaves, betel, Imlay, kaduk, and stinkvine are shown in
Antibacterial activity
Infectious diseases caused by drug-resistant bacteria are a worldwide concern, and plants are a natural source of many biological compounds with potential antibacterial properties54, 55. The antibacterial activities of different fractions, such as n-hexane (HE), ethyl acetate (EA) and water (W), from bitter leaves, betel, Imlay, kaduk, and stinkvine are shown in
This may be because betel leaves contain antibacterial compounds, even those against multidrug-resistant bacteria, such as hydroxychavicol, stearic acid, and palmitic acid 56. According to Muruganandam 57, high contents of phenols and flavonoids can impart high inhibitory effects on microorganisms. However, the biological activity of these compounds is strongly dependent on the chemical nature and polarity of the extraction solvent. Haminiuk 58 demonstrated that phenolic and flavonoid contents are significantly lower when these compounds are extracted with hexane. Therefore, the antibacterial ability of hexane extracts is also more limited than that of extracts from other polar solvents, such as water, methanol, ethyl acetate and ether, from betel leaves. These results are similar to those of the study by Armansyah . 59 on the antibacterial activity of the EA fraction from red betel leaves, which revealed that the EA fraction has a broad spectrum of antibacterial activity against all tested microorganisms (, and ).
Conclusions
In this study, the results showed that all five plant extracts were good sources of natural antioxidants and antibacterial agents. The total phenolic content and antioxidant activities (DPPH, ABTS, and FRAP) of the extracts from the five compared leaves showed that betel leaves had the highest activity, while Stinkvine and Imlay had the lowest activity. The correlations between TPC and FRAP and between DPPH and ABTS were quite close, with all correlation coefficients greater than 0.92. These findings suggested that phenolic compounds play a major role in the antioxidant activity of FRAP, ABTS, and DPPH. Among the five leaf extracts, the Betel leaf extract had the best antioxidant and antibacterial activity. Moreover, bitter leaves had the lowest antibacterial activity. This shows that the biological potential of fractionated solvent extraction from five types of leaves is very large and has many applications in different fields.
Acknowledgments
The authors would like to thank Nguyen Tat Thanh University for permission and for providing facilities during the research period.
Author Contribution
Tuyet-Ngan Lien: Investigation; Data curation; Writing - original draft. Tran-Phong Nguyen: Conceptualization; Investigation; Writing - original draft. Quoc-Duy Nguyen: Investigation; Writing – original draft. Nhu-Ngoc Nguyen: Conceptualization; Data curation; Investigation; Methodology; Writing - original draft; Writing - review & editing. All authors read and approved the final manuscript.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding statement
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Conflict of interest disclosure
The authors have no relevant financial or non-financial interests to disclose.

