Synthesis of a unique oligomer by means of the thiol-maleimide reaction of extending 1,1'-(methylenedi-4,1-phenylene) bismaleimide chains for the employment of self-healing polymers
- National Key Laboratory of Polymer and Composite Materials– Ho Chi Minh City, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam
- Vietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc City, Ho Chi Minh City, Viet Nam
- Faculty of Materials Technology, Ho Chi Minh City University of Technology, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam
Abstract
Introduction: The Diels-Alder reaction of maleimide and furan groups is being exploited in self-healing materials; hence, it is no wonder that with the ownership of the former, 1,1'-(methylenedi-4,1-phenylene) bismaleimide (BMI) counts as a potential candidate in this area. However, it displays certain discernible demerits arising from its inflexible structure. Furthermore, the maleimide group, in all likelihood, easily takes part in the Michael addition reaction with thiol groups under basic conditions. It is crucial that this element opens the door for this ingredient to be amended with a view to weathering its shortcomings. The goal of this research is to modify the structure of BMI to create a new oligomer with maleimides at the end of its chains.
Method: The oligomer was synthesized by means of the reaction of the maleimides of BMI and the thiol groups of 2,2’-(ethylenedioxy) diethanethiol (dithiol) with the support of triethylamine (TEA). In addition, an investigation into the possibility of this reaction happening without the base catalyst was carried out. The application of a wide assortment of modern measurements ranging from 1H-NMR and FT-IR to GPC provided some useful data about the molecular weight and whether the reaction occurred.
Result: According to the information, the formation of the oligomer owning the maleimide and thiol terminal functions was observed with the help of TEA over the 24-hour timescale at room temperature, whereas that with a lack of this catalyst is impossible.
Conclusion: The research will make a major contribution to the creation of new healable polymers reliant on the Diels-Alder mechanism in the foreseeable future.
INTRODUCTION
It is courtesy of its reversible ability that the Diels-Alder reaction from maleimide as well as furan groups has incorporated into catholic self-healing materials; it is likely to be triggered in the environment of under <100 °C1. Thanks to this, being destroyed, materials are apt to recover their initial properties with the assistance of this reaction- indeed, the reformation of damaged Diels-Alder links would recur at the temperatures. Therefore, the occurrence of deep cracks would be eliminated2. There is no surprise that a large amount of research has utilized this unique reaction to engender the high self-healing performance of polymers3, 4, 5, 6, 7.
Not only is 1,1'-(methylenedi-4,1-phenylene) bismaleimide (BMI) referred to as a heat-resistant resin, but it also possesses astounding mechanical strength because of the presence of aromatic rings in its structure together with the high crosslink density8. However, this can be seen as a double-edged sword inasmuch as these features contribute to making BMI-based materials fragile9. By mounting the gap between maleimide groups to drain their crosslink density and help molecular chains rotate in a free way with more space, this tangible drawback can be overcome9. In a strenuous bid to do so, not surprisingly, BMI scores modified with various groups, such as biphenylene10, phosphorous 11, naphthalene12, 13, and 1,3,4-oxadiazole14, have been illustrated in numerous studies. Additionally, the utilization of the thiol-maleimide reaction would be beneficial for integrating other chemical substances into BMI. The root behind this is that it has been viewed as the most efficacious Michael addition reaction since time immemorial15, 16. In addition, maleimide groups are capable of effortlessly participating in this reaction because the reactive carbonyl groups quicken the occurrence of the reaction. Furthermore, the supplementation of catalysts such as dimethylphenylphosphine 17 and triethylamine18 is theorized to somewhat facilitate the thiol-maleimide Michael reaction. In particular, the base first attacks thiol groups, forming thiolate anions18. Then, they react with activating C=C bonds in the maleimide structure, bringing about the Michael adduct and new thiolate anions paving the way for the subsequent reaction.
In this research, we concentrate on the synthesis of new BMI-based oligomers by the thiol-maleimide Michael reaction. Herein, 1,1'-(methylenedi-4,1-phenylene) bismaleimide (BMI) was reacted with 2,2’-(ethylenedioxy)diethanethiol (dithiol) in the presence and absence of triethylamine. Consequently, the results from FT-IR, H-NMR, and GPC measurements validated the success of this reaction and the formation of the newly synthesized oligomer. It is due to boasting residual maleimides in its molecular chains after the reaction that this oligomer can be made use of the synthesis of Diels-Alder healable polymers through step-growth polymerization with other oligomers comprising Furan groups. This pragmatic approach was applied in other studies19, 20. Obviously, the DA reaction is reversible under heating conditions. In detail, it is because the DA adduct is exposed to a suitable thermal environment that the retro-DA reaction occurs with the fracture of covalent linkages. In contrast, the recurrence of the [4+2] cycloaddition DA reaction and the reformation of the bonds are documented at lower temperatures21. Furthermore, maleimide groups participate in this reaction in a febrile way due to a scarcity of C=C bond electrons22. From their sound, the application of oligomers would, more or less, facilitate the self-healing ability of polymers out of the assistance of the DA reaction.

The synthesis of the BMI-based oligomer via the reaction of 1,1'-(Methylenedi-4,1-phenylene) bismaleimide and 2,2’--(Ethylenedioxy) diethanethiol with the assistance of TEA.
EXPERIMENTATION
Materials
2,2’-(Ethylenedioxy)diethanethiol (95%, Sigma‒Aldrich), 1,1'-(Methylenedi-4,1-phenylene)bismaleimide (95%, Sigma‒Aldrich), Trichloroacetyl isocyanate (TAI, Acros), Triethylamine (TEA, Acros), n-Heptane (Fisher), Chloroform (CHCl, Fisher).
Characterization
A Bruker Advance 500 MHz spectrometer was used to obtain H-NMR results indeed, we took advantage of CDCL and tetramethylsilane as the solvent and an internal reference, respectively. An FT-IR Bruker Tensor 27 served as a tool to obtain transmission Fourier transform infrared (FT-IR) spectra via a KBr disk at a 4 cm resolution after 256 scans. The Polymer PL-GPC 50 gel equipment with a detector of RI was harnessed to secure GPC results at a flow rate of the THF eluent (1 mL/min). The molecule-related weight along with its distribution (D) were calculated as per the criteria of polystyrene.
Synthesis of polysulfide
Initially, the CHCl solvent was chosen to wholly dissolve BMI with the ratio of CHCL to it (3 ml/100 mg) in an ultrasonic bath prior to mixing them with 2,2’-(ethylenedioxy) diethanethiol. Next, the TEA catalyst was added to the homogeneous mixture. This reaction occurred at room temperature during the 24-hour period. In a subsequent phase, the N-heptane solvent was applied to enable the entity to precipitate before being put into the vacuum system to eradicate the emergence of solvent in the G1 sample. With respect to the specimen known as G2 with a TBP vacuum, the process of synthesizing it comprises all the aforementioned steps except the addition of TBP.
RESULTS
G1 was obtained through the reaction shown in Figure 1. Furthermore, the information of G1 and G2 is presented in
Detailed information on G1 and G2
|
Product |
Ratio (BMI/Dithiol) |
TEA (%wt) |
|
G1 |
3:2 |
1 |
|
G2 |
3:2 |
0 |
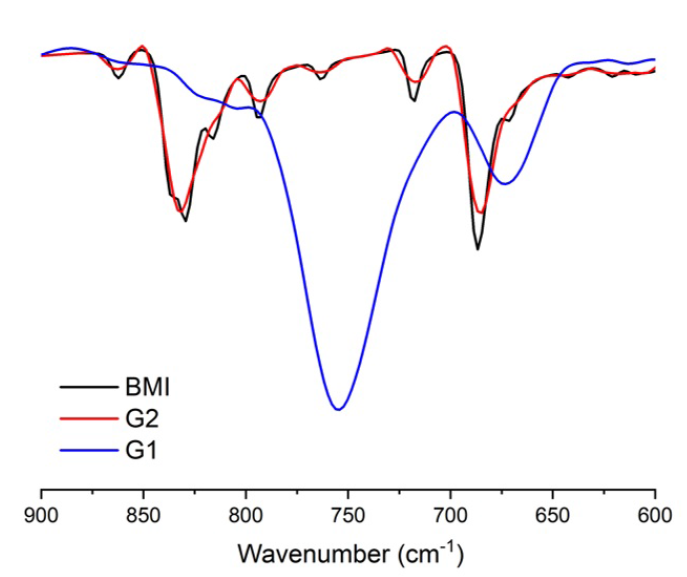
In regard to their FT-IR spectra in Figure 2, the two samples possess two special peaks at 828 cm and 690 cm; those of BMI witnessed an identical trend. They are ascribed to the vibration of maleimide C-N-C groups stretching together with the framework correspondingly. With respect to the results in Figure 3, there is a decided peak at 2557 cm stemming from SH function stretching8, 17. Notably, all G1, G2 and dithiol bear this peak.

The FT-IR spectra of BMI, G2 and G1.

The FT-IR spectra of dithiol, G2 and G1.
Represented in Fig 3 is the GPC result showing the molecular weight of G1. Obviously, the Mn and PDI of G1 are recorded at 1765 g/mol and 2.8, respectively.

GPC curve of G1.

The 1H-NMR spectrum of BMI.

The 1H-NMR spectrum of dithiol participating in the reaction with TAI in the solvent.

1H-NMR spectrum of G1 joining in the reaction with TAI in the solvent CDCl3.
The 1H-NMR spectra of BMI, dithiol, and G1 reacting with TAI are shown in Figure 5, Figure 6 and Figure 7, respectively. It is obvious that 6.83 ppm is the peak of maleimides in Figure 523. Additionally, after reacting with TAI, the thiol groups of dithiol show a manifest peak at 9.45 ppm. Furthermore, in Figure 6, the peak of G1 at 6.83 ppm is characterized by maleimides, proving the emergence of maleimides at the end of molecular chains. In addition, G1’s thiol groups reacting with TAI shed light on their peak at 8.5 ppm in lieu of 9.45 ppm in view of 9.45 ppm similar to that of these functions in Figure 7. The reason behind this is that the former is positioned at the end of the high molecular compound23.
DISCUSSION
The new oligomer based on BMI was synthesized by the reaction of dithiol and BMI via the thiol-maleimide Michael mechanism. It took 24 hours to take place at room temperature with a ratio of these ingredients of 3:2. It is the study that focused on drawing a comparison between the happenstance of the reaction with the help of TEA – a base catalyst – and that with a want of it. This condition was applied in a previous study18.
Looking at the FT-IR spectra more closely, we are disposed to swiftly observe that the intensity of G1’s maleimide peaks at 828 and 690 cm is lower than those of the 1'-(methylenedi-4,1-phenylene) bismaleimide peaks. It is notable that its thiol peak also witnessed an analogous trend with a decrease in its intensity. It follows that the foregoing peaks did not evaporate completely after the reaction, substantiating the occurrence of these groups at the end of the G1’ molecular chains. Conversely, scarcely was the decrease in the intensity of these groups in G2 documented, thanks to which, the reaction is postulated to hardly happen. This phenomenon did prove an indispensable role TEA must play in making the reaction possible. It comes as no surprise that there is no need to conduct any further in-depth analysis of G2.
For the GPC result of G1, the molecular weight distribution curve started from the value of 200. This is a strong likelihood that there are some residual thiol groups in line with its FT-IR outcome. In addition, the molecular weight is 1765 g/mol, following which the formation of the new high molecular compound was detected.
According to G1’s 1H-NMR peaks, the reason behind the shift of the thiol peak from 9.45 ppm to 8.5 ppm is the emergence of thiol terminal groups. The reaction of BMI and dithiol is confirmed to take place to form longer molecular chains because of the peaks at 2.64, 2.88 and 3.21 ppm exhibiting the linkage between them24.
It is a reality that the strength of our means of synthesizing the oligomer from the Michael addition reaction is undisputed. The reason behind this is that our reaction could happen without the support of external factors such as UV light accompanied by heat. The obvious merit makes the process of synthesizing our oligomer simple and easy to conduct. Compared to other studies, our research excels to some extent. In fact, by performing the Michael addition click reaction between methacrylate-polyhedral oligomeric silsesquioxane and thiol-containing substances in a range of 45-55 °C in the presence of a phosphine-based catalyst, dimethylphenylphosphine, Hui Lin successfully formed macroporous hybrid monoliths25. Moreover, Weixian Xi designed a network of polymers by means of the thiol-Michael reaction of tetrathiols and bisphenol A ethoxy diacrylate under UV conditions with the aid of a photocatalyst of 2-(2-nitrophenyl)propyloxycarbonyl-hexylamine over a 30-minute period26. In spite of the aforementioned upside, the research shows its shortcomings. It is necessary to spend 24 hours for the reaction to take place. The amount of time in this research is larger than that of previous studies25, 26.
CONCLUSIONS
The BMI-based oligomer was successfully synthesized via the thiol-maleimide reaction of 1,1'-(methylenedi-4,1-phenylene) bismaleimide and 2,2’-(ethylenedioxy)diethanethiol in the presence of TEA. In contrast, the reaction of synthesizing G2 without TBP hardly occurred. According to FT-IR, GPC and 1H-NMR results, the emergence of the high molecular compound and the formation of the relationship between the chemical substances were pointed out. G1’s GPC curve illustrates its Mn of 1765 g/mol. Strikingly, G1 possesses maleimide groups at the end of molecular chains. This feature would contribute to the application of this oligomer in the syntheses of self-healing polymers.
Acknowledgments
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number 562-2022-20-01.
LIST OF ABBREVIATIONS
FT-IR: Transmission Fourier transform infrared
H-NMR: Proton nuclear magnetic resonance
GPC: Gel permeation chromatography
Mn: Number average molar mass
AUTHOR CONTRIBUTION
Nguyen Khai Hoang Nguyen, Mai Ly Nguyen Thi: methodology, investigation, Duc Anh Nguyen Song, Huan Hoang Dang, Thuy Thu Truong: investigation, formal analysis, Le-Thu T. Nguyen: supervision.

