A healable thermoset possessing shape-memory effect assisted recovery efficiency via thiol-click and diels-alder reaction between furanic groups and bismaleimide crosslinker
- National Key Laboratory of Polymer and Composite Materials – Ho Chi Minh City, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam
- Faculty of Materials Technology, Ho Chi Minh City University of Technology, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam
Abstract
Introduction: This study elucidates a convenient and uncomplicated synthesis methodology for acquiring novel thermally self-reparable polymer materials exhibiting concurrent efficiency in healing and commendable mechanical properties. This concept was developed through the utilization of the "thio-click" mechanism and the Diels-Alder reaction between polycaprolactone-bisfuran and bismaleimide to produce preliminary products, employing tris-furanic acid as a crosslinker reagent. Investigate the mass ratios of two precursors, PCL-bisfuran and trisfuran, to find the optimal material system (evaluated based on mechanical properties, shape memory, and self-healing capabilities).
Methods: In this research, the intermediate products and final material were characterized via proton nuclear magnetic resonance (1H-NMR) to accurately identify the chemical structures. Tensile strength machinery was used to record and evaluate the healing efficiency. The obtained network exhibited self-recovery damage ability under mild temperatures via optical microscopy and tensile analysis.
Result: This study was successfully performed due to obtaining accurate product chemical structures, showing a mechanical recovery efficiency of 70–80% and good crack healing at 70 °C in 30 minutes.
Conclusion: This concept was intended to partially contribute to the advancement of self-healing polymer research and its applications in various fields.
INTRODUCTION
Self-healing materials have been studied, developed, and attracted enormous attention from the scientific community due to their ability to repair fractures before thorough failure. The presence of microcracks can easily change the properties of the final materials. Thus, this potential material has become an important research field in the study of thermosets in the past decades1, and it has made a solid foundation to improve the service lifetimes of materials and lower the cost in ranges of attractive applications such as anti-corrosion coatings, electronic skin, and biosensors2, 3, 4, 5, 6.
Generally, healable material is classified into intrinsic and extrinsic mechanisms 1. Intrinsic healing is considered a productive method due to its high self-healing efficiency and repeatable ability to heal damage at the same position, which includes covalent bonds and supramolecular interactions7, 8, 9. Among these approaches, reversible covalent bonds are more attractive. The most popular reversible interaction is the Diels-Alder (DA) reaction between the furan and maleimide groups, which is thermally reversible10, 11, 12. The conversion between DA and the retro DA reaction could be obtained when heating up and cooling down 90 °C 13.
Several groups have studied bearing furan/maleimide functionalities as pendant groups to incorporate DA (Diels-Alder) bonds into their structure. However, these systems often face a conflicting priority between mechanical strength and the required high mobility of dynamic bonds for effective healing14, 15, 16, 17. Previous studies commonly employed the hard/soft multiphase architecture of polyurethanes (PU) and integrated DA bonds into the hard phase. This result is low healability due to its limited mobility12, 18, 19, 20, 21. To address this drawback, this study will improve the method to ensure not only good mechanical properties but also high self-healing ability at low temperatures (70 °C). A polymer structure possessing healing ability via Diels-Alder reversible bonds was synthesized from 2 precursors, which are bisfuran-terminated polymers containing two furan moieties at the polycaprolactone chain ends and (thio)urethane-trisfuran. Then, the intermediate product was crosslinked by the D-A reaction of furanic groups and bismaleimide. The modified structure with dynamic DA moieties at the end of PCL chains offers reduced microphase separation and improved dynamic properties compared to traditional polyurethanes/polyureas. This unique architecture provides greater control and flexibility, making it a promising approach in self-healing materials. Concentrating on the synthesis of polymers, the “thiol-click” reaction is a potential method to reach a high reaction yield, simply react and easily eliminate byproducts without chromatography. A wide range of these reactions includes thiol-ene, thiol-yne, thiol-isocyanate, and thiol-epoxy reactions22. Polycaprolactone was used, which is a linear semicrystalline to provide the shape recovery effect under trigger temperature, which can keep scratch surfaces closer to allow healing without external effects23.

Demonstration of the chemical structures of the obtained network.
Scheme 1 illustrates the step-by-step synthesis of a polymer structure with healing ability through Diels-Alder reversible bonds. The process involves the use of bisfuran-terminated polymers, (thio)urethane-trisfuran, and bismaleimide to create a modified structure for self-healing materials with high performance even at low temperatures.
EXPERIMENTAL
Materials
Polycaprolactone-diol (PCL, Mn=8000) was received from Pertorp, and hexamethylene diisocyanate dimer (HDI dimer), furfuryl thiol (97%), bismaleimide (1,1’-methylenedi-4,1-phenylene), hexamethylene diisocyanate isocyanurate trimer (Desmodur® N 3390 BA), and triethylamine (TEA) were purchased from Sigma‒Aldrich. Solvents (chloroform, toluene, n-heptane, dimethyl formamide) were provided by Fisher-Acros. Zirconium (IV) acetylacetonate (Merck, Germany) was used as the catalyst.
Characterization
Proton nuclear magnetic resonance (H-NMR) spectroscopy was measured by a Bruker Advance 500 MHz spectrometer using deuterated chloroform (CDCl) with TMS as an internal reference. Tensile strength results were recorded on a tensile testing machine (Tensilon RTC-1210A). The sample met the requirements of ASTM D882. Optical microscopic images were obtained on an Olympus GX51F microscope.
Synthesis of precursors bisfuranic polycaprolactone
The reaction takes place in 2 stages. In the first step, polycaprolactone diol was azeotropically dried and added to the flask. Then, hexamethylene diisocyanate was slowly dropped into the reaction. Zirconium(IV) acetylacetonate was dissolved in anhydrous chloroform. The mixture was stirred and refluxed at 65 °C for 4 hours. In the next step, the reaction system was cooled down. Furfuryl thiol and TEA were added and stirred at room temperature for 24 hours until homogeneous. All operations above were carried out under a nitrogen atmosphere. The final mixture was evaporated to remove the solvent and then precipitated by n-heptane multiple times to eliminate the unreacted furfurylthiol. The sample was dried and obtained under vacuum.23, 24
Synthesis of precursors (Thio)urethane-trisfuran
The procedures for synthesizing a (thio)urethane network including three furan moieties were successfully conducted and published in previous studies. The mechanism of this system is a “click” thiol–isocyanate reaction between HDI-trimer and 2-furfurylthiol25.
Synthesis of the self-healing material formed from PCL-bisfuran, (thio)urethane-trisfuran, and the crosslinker bismaleimide.
Bisfuranic polycaprolactone, Trisfuran, and the crosslinker Bismaleimide were dissolved in DMF. The equivalent ratio between the furanic group and Bismaleimide is 1:1. The massive ratio between bisfuran and trisfuran was investigated at 6:1. The mixture was stirred and then poured into a silicone mold at 60 °C to crosslink the network. The product was vacuum dried and obtained.
RESULTS
Proton nuclear magnetic resonance (H-NMR)
The 2-step procedure of the bisfuran-terminated polymer possessing two furan moieties at the polycaprolactone chain ends was synthesized via 2 reactions. The “click” mechanism (alcohol-isocyanate reaction) between PCL (Mn = 8000 g/mol) and HDI dimer with zirconium (IV) acetylacetonate as the catalyst and the other is the thio-isocyanate reaction. The first-step product reacted with furfurylthiol using TEA as the catalyst26. The structure of this polymer was characterized by proton nuclear magnetic resonance (1H-NMR) spectroscopy (Figure 2). The peak u at 4.74 ppm and peak w at 5.4-5.6 ppm come from the appearance of OCO-NH and SCO-NH groups. Other peaks (a, b, c, d) indicate the existence of furan functionality23.
Shape-memory capability
The shape-memory ability of the obtained product is shown in Figure 3. The sample was deformed to a tensile or spiral shape after heating to 70 °C, and the temporary shape was kept fixed by cooling to a cold temperature for 3 minutes. Then, after reheating the sample to 70 °C, the original shape was completely obtained within 15 seconds. The test was conducted in 3 cycles to record the recovery time after each cycle at the same temperature. In the third cycle, the recovery time was 20 seconds, which is 5 seconds above the first one.

1H-NMR (500 MHz, CDCl3) spectra of PCL diol (A) and bisfuranic PCL(B).

The shape-memory ability of the Diels-Alder polymer.
Self-healing performance via optical microscopy
The results of sequential healed performances are shown in Figure 4 and Figure 5. The self-healing ability of the material was followed by optical microscopy. The damage was applied to the sample surface with a razor blade. The healing performance was investigated by heating the sample at 65 °C and 70 °C.

Optical microscopy of scratch healing at 65 °C for 0 hours (a), 30 min (b), 60 min (c), and 2 hours (d)

Optical microscopy of scratch healing at 70 °C for 0 min (a), 15 min (b), 30 min (c), 60 min (d)
Figure 4 shows that the scratch nearly disappeared after 24 h at 65 °C. In addition, while setting the temperature up to 70 °C, the healing process finished within only 30 minutes, with an insignificant scar, and it completely disappeared in 60 minutes - a half compared to healing at 65 °C (Figure 5).
Self-healing performance via mechanical properties

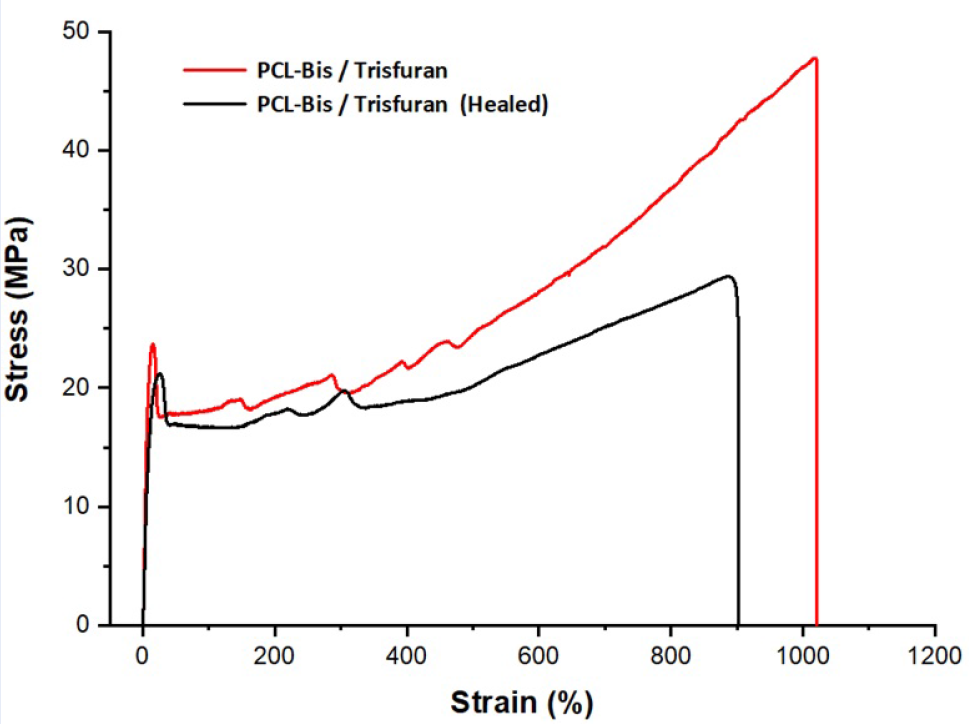
Stress‒strain curves of the initial and healed samples.
The cracked-healing efficiencies of the sample were followed by mechanical properties (tensile strength), and the performance results are shown in Figure 6 and
Tensile strength of the initial sample and cut sample after healing at 70 °C
|
Properties |
Initial |
Healed |
Healing Efficiency (%) |
|
Young’s modulus (MPa) |
284.6 |
216.05 |
75.91 |
|
Stress at break (MPa) |
47.784 |
28.406 |
59.44 |
|
Strain at break (%) |
1016.9 |
898.52 |
88.36 |
The samples exhibited remarkable mechanical properties, with Young’s modulus of both the initial and healed material surpassing 200 MPa (
DISCUSSION
The H-NMR spectra of PCL diol (A) and bisfuranic PCL provide valuable information about the successful synthesis of bisfuran-terminated polycaprolactone. The appearance of peak u in the H-NMR spectra indicates the successful incorporation of a urethane group into the polymer structure. This urethane group is typically formed by the reaction between an isocyanate and an alcohol, suggesting that the polymer has undergone a reaction involving an isocyanate and the hydroxyl groups of the PCL diol. Furthermore, the presence of peak w signifies the successful incorporation of a thiol group into the polymer structure. In addition to the urethane and thiol groups, the bisfuranic PCL polymer also possesses furan functionality in its main chains. The presence of furan groups in the polymer structure can be confirmed by analyzing the characteristic peaks corresponding to furan protons (a, b, c, d) in the H-NMR spectra. Overall, the H-NMR spectra of bisfuranic PCL demonstrates the successful synthesis of a polymer with bisfuran-terminated polycaprolactone fully containing urethane and thiol groups, as well as furan functionality in its main chains. These spectroscopic findings provide valuable insights into the composition and structure of the polymer.
Talking about examining the shape-memory capability of the studied polymer, it is clear that this material can remember the tempotory form and recover to the original shape. This observation can be attributed to the incorporation of PCL segments into the structural composition. Due to its thermoplastic nature, remarkable elasticity, and melting temperatures typically ranging from 45 to 67 °C, PCL has a shape-memory ability, making it an attractive choice for use as a switching element. On the other hand, we noticed that the recovery time tended to increase after each cycle. This can be explained by the fact that after each strain, some bonds in the molecular chain may have been partly broken. Furthermore, some molecular chains of polycaprolactone tended to rearrange in a certain order, so the crystallization in the PCL phase increased insignificantly. As a result, the recovery rate decreases gradually after cycles23, 25
The self-healing performances of the polymer via optical microscopy at 65 °C and 70 °C were evaluated. Scratches were induced by damaging the surface using a scalpel blade. Figure 4 and Figure 5 display microscopic images of the surface damage before and after the healing process. The DA-based material exhibited complete recovery of the scratches after 2 hours at 65 °C and 1 hour at 70 °C. The surface showed almost no visible traces of the previous scratches. The difference in healing time is considered the elevation of temperature to 70 °C, which enhances the flexibility of the PCL segments within the polymer structure. This increased flexibility enables better molecular alignment and reorganization. Consequently, the healing process becomes more effective in a short time or even complete elimination of surface scratches.
The reason why those 2 temperatures were chosen was that the polycaprolactone chains become more mobile and flexible at temperatures above 60 °C. Furthermore, the high density of entanglements between the permanent crosslinks contributes to a strong driving force for the recovery of the network. This interplay between mobility and entanglement density facilitates the shape memory effect, enhancing healing efficiency. Thus, 2 scratch surfaces could be kept closer to each other so that the reaction between the furan and bismaleimide moieties at the damage interface could easily take place.
According to the result from the stress‒strain curve of the polymer, the material was successfully generated, ensuring the mechanical property for other application requirements. There are several reasons to explain why modulus recovery is good. First, the hard phase containing hydrogen bonds between the urethane and (thio)urethane groups may be improved. The second is the conversion of DA adducts resulting in the formation of well-built hydrogen bonds in the (thio)urethane network. In addition, heating samples above Tg can provide shape-memory effects of molecules in polycaprolactone chains and increase the mobility of the network, which makes it possible for the DA reaction to occur and form a crosslinking network.23, 24, 25
CONCLUSION
The study successfully achieved its objective of proposing and investigating a method to create materials that can heal under moderate conditions without conflicting with their mechanical properties. This was achieved by incorporating dynamic Diels-Alder and thiourethane bonds into the materials, which enabled shape recovery and healing abilities. These materials demonstrated not only the capability to heal scratches at mild temperatures but also good tensile strength recovery.
A concept was successfully synthesized by crosslinking maleimide poly(caprolactone-thiourethane) precursors that contained multiple rigid bismaleimide segments. The chemical structure of the product was accurately obtained via H-NMR spectroscopy and showed a mechanical recovery efficiency of 70–80% and good crack healing at 70 °C in 30 minutes. The suitable temperature for testing shape memory and self-healing ability was above 60 °C due to the mobility of the well-crystallized crosslinked PCL segments and an active reformation of the DA bonds.
By examining the mass ratios of two precursors, PCL-bisfuran and trisfuran, it is possible to achieve a favorable combination of mechanical performance and dynamic molecular reversibility, leading to enhanced self-healing capabilities by adjusting the network architecture. This advancement opens possibilities for flexible modifications in polymeric systems, which could serve as inspiration for the creation of high-performance self-healing materials.
Acknowledgments
This research is funded by Vietnam National University Hochiminh City (VNU-HCM) under grant number 562-2022-20-01.
LIST OF ABBREVIATIONS
FT-IR: Transmission Fourier transform infrared.
H-NMR: Proton nuclear magnetic resonance
PCL: Polycaprolactone
DA: Diels-Alder reaction
TEA: Triethylamine
Mn: Number average molar mass
AUTHOR CONTRIBUTION
Huan Hoang Dang, Mai Ly Nguyen Thi: methodology, investigation, Duc Anh Nguyen Song, Nguyen Khai Hoang Nguyen, Thuy Thu Truong: investigation, formal analysis, Le-Thu T. Nguyen: supervision.
Competing interests
The authors declare that they have no competing interests.

