Study on the formation and growth of white turmeric rhizomes (Curcuma aromatica Salisb.)
- University of Science, VNU-HCM
Abstract
White turmeric has underground stems that develop rhizomes. It has been used as an Eastern traditional herb for a long time. As with many other monocotyledons, the growth of white turmeric is mainly due to the presence of the primary thickening meristem. Currently, the study of the primary thickening meristem, radial growth, and accumulation of secondary material as essential oil in turmeric rhizomes, including white turmeric, is still limited. In this study, we observed the anatomical structural changes in white turmeric in early growth stages in vitro: we counted the number of PTM layers, the number of vascular bundles, and the number of essential oil drops in the rhizomes and determined physiological and biochemical indicators, such as respiratory intensity, tuber diameter, fresh weight, dry weight, total sugar content, and starch level, according to each stage of early growth (0, 2, 4 and 6 weeks) of white turmeric. The results showed that at 0 weeks of age (resting buds), the PTM consists of 1-2 layers of cells, and there is no formation of vascular bundles and essential oils in tubers. At 2 weeks of age, the PTM consists of 6-7 layers of cells, and there is the formation of vascular bundles and the appearance of essential oil droplets in the parenchyma. The number of PTM cell players decreases to 3-4 layers at 4 weeks of age and 2-3 layers at 6 weeks of age, but the tuber diameter and the number of vascular bundles increase rapidly. Along with an increase in fresh weight, dry weight, starch content, and the number of drops of essential oils, there was a decrease in the sugar content and respiratory intensity in the rhizome. The relationship between the number of PTM layers, the number of vascular bundles and diameter, sugar content, starch, and the number of essential oil droplets was also discussed.
INTRODUCTION
White turmeric Salisb. is a monocotyledon that has been used traditionally for the treatment of rheumatism, menstrual disorders, wound healing, and antibacteria in Eastern India, China, and Vietnam for a long time1, 2, 3. A special meristem in the limit zone between the cortex and the vascular cylinder is associated with the rhizome radial growth of white turmeric. It is called the “primary thickening meristem” (PTM) by many authors4, 5, 6. The circumferential growth of white turmeric through the primary thickening meristem leads to an increase in the size as well as the ability of the tuber to contain essential oils, thereby increasing the yield of white turmeric. In Vietnam, studies of growth and the effect of plant hormones in white turmeric rhizomes are still limited. There is a study of white turmeric rhizome formation by MS, BA, and sucrose7. Most studies target white turmeric secondary compounds as the extraction of sesquiterpenoids in white turmeric, including furanodiene, furanodienone, curzerenone, germacrone, curcumenone and zederone8, 9, 2. Some studies have targeted medical applications, such as the simultaneous quantification of cordon germacrone in white turmeric extract and the antibacterial effect of white turmeric extract10, 11, 12. In this study, we targeted primary thickening meristem activity in the radial growth of white turmeric rhizomes ( Salisb.), to contribute to increasing the size, accumulation, and yield of white turmeric thanks to the operation of this PTM.
MATERIALS AND METHODS
Plant material
The rhizomes of Salisb, which have 5 – 6 buds, were collected on a farm at the Daklak Center for Plant and Animal Breeding 12 months after planting.
Study on white turmeric growth
To remove dust, fungal spores, and bacteria, the rhizomes of Salisb with buds were first washed thoroughly with a soak in running tap water and then treated with 70% alcohol for 2 min 3 times. Then, the explants were treated with Javen 35% solution for 10/10/15 min followed by 3 washes. After that, it was processed with 0.1% HgCl for 12 min and washed with sterile distilled water 4 times. Each piece with one bud (10 mm) was placed on MS culture with cotton wool. The growth of buds was determined after each 2, 4, and 6 weeks by diameter, dry and fresh weight, respiratory intensity, total sugar and starch content in rhizomes and anatomy structure: cell - layers of PTM, number of vascular bundles, and essential oil drops.
Biochemistry and physiology analysis
Rhizome anatomy was observed directly through an optical microscope. Anatomical sections were cut longitudinally and transversely and stained with Lugol's iodine and indigo carmine solution. Essential oil droplets in the cross-section of maximum diameter were counted directly on the unstained section
Fresh weights were recorded immediately after harvesting. Dry weight was recorded after drying rhizomes at 120°C per hour and then at 80°C to constant weight (approximately 72 hours at 80°C).
The rhizome diameter was determined directly at the maximum rhizome diameter using a caliper.
The respiratory intensity was measured using the Hansatech Leaf Lab 2 device. A 0.5 cm longitudinal-to-apical section was placed in the apparatus without illumination at 26°C, and oxygen uptake (µmol O/g fresh weight/min) was measured.
At each stage, 1 g of fresh rhizome weight was harvested and extracted with ethanol (3 times with 70% ethanol and finally with 90% ethanol), and the supernatant was collected to determine sucrose content. The starch content pellets were dried and hydrolyzed with perchloric acid to collect and determine the starch content. The sucrose concentration was determined by optical density measured at a wavelength of 490 nm using 5% concentrated phenol sulfate. The starch concentration was obtained by multiplying the conversion factor by 0.9 13.
A completely randomized design (CRD) with 10 replicates was used as the experimental design. Data were analyzed using the Statistical Package for Social Sciences (SPSS) software version 16.0 for Windows. Analysis of variance (ANOVA) was used to test for significant differences. Mean separation tests were performed using Duncan's test.
RESULTS
At the 0-week stage, the buds are still under the leaf scales of the mother rhizome. At the 4-week-old stage, the tips of the buds are surrounded by many leaves, and the rhizomes are quite large in diameter. At the 6-week stage, a pseudostem is formed, and the rhizome is probably pear-shaped (Figure 1).

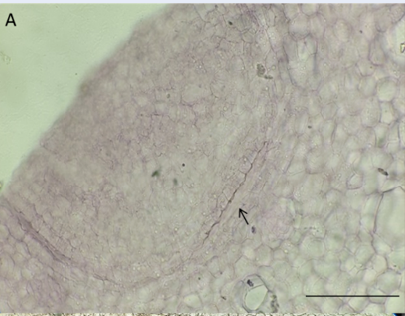
Early stages of white turmeric bub growth

Longitudinal sections through the shoot apical meristem: A. 0-week stage, B. 2-week stage, C. Zoom at PTM, D. 4-week stage, E. 6-week stage. The ruler is 200 µm.

Transverse sections of the largest diameter: A. 0-week stage, B. 2-week stage, C. 4-week stage, D. 6-week stage. The ruler is 200 µm.

The hypothesis of the model about the activity of PTM and radial growth in white turmeric.
In a longitudinal section across the shoot apical meristem at the 0-week stage, the bud has a leaf primordium envelope of the shoot apical meristem (SAM). The pith and cortex were disgusting by one to two layers of cells with tangentially flattened cells rising downward from the SAM. At the 2-week stage, buds emerge with the apical meristem primordial sheath. The primary thickening meristem (PTM) is located between the pith and cortex and has 6-7 tangentially flattened cell layers. At 4 weeks, the pith becomes larger than the cortex. At the 6-week stage, the pith becomes much larger than the cortex in comparison to the 4-week stage (Figure 2).
Rhizome diameter, vascular bundles, and cell layers of PTM in white turmeric tubers
|
Week stages |
Rhizome diameter (mm) |
Cell layers of PTM |
Vascular bundles |
|
0 |
0.77 ± 0.06d |
1.33 ± 0.33c |
- |
|
2 |
1.91 ± 0.06c |
6.33 ± 0.88a |
63.33 ± 2.40c |
|
4 |
3.65 ± 0.04b |
3.67 ± 0.33b |
94.67 ± 3.52b |
|
6 |
5.58 ± 0.11a |
2.67 ± 0.33bc |
106.67 ± 7.52a |
Fresh weight, dry weight, respiratory intensity, and essential oil drops in white turmeric rhizomes
|
Week stages |
Fresh weight (g) |
Dry weight (mg) |
Sugar content (mg/g TLK) |
Starch content (mg/g FW) |
Essential oil particles (drop) |
Respiratory intensity (µmol O2/g/phút) |
|
0 |
0.15 ± 0.01d |
2.41 ± 0.34d |
0.82 ± 0.12d |
0.51 ± 0.08a |
- |
1.81 ± 0.04a |
|
2 |
0.28 ± 0.01c |
14.83 ± 0.19c |
76.04 ± 0.31a |
42.04 ± 0.25b |
22,67 ± 1.20c |
0.41 ± 0.13b |
|
4 |
0.35 ± 0.01b |
36.11 ± 0.12b |
28.22 ± 0.23b |
82.64 ± 0.12c |
113.33 ± 4.33b |
0.21 ± 0.09c |
|
6 |
0.40 ± 0.02a |
37.72 ± 0.13a |
15.06 ± 0.16c |
191.48 ± 0.28d |
253.00 ± 6.81a |
0.19 ± 0.07c |
There were no vascular bundles or essential oil droplets in the 0-week maximum diameter section. There is a distinction between the pith and cortex by 1 – 2 tangentially flattened cell layers. At the 2-week stage, PTM occurs between the pith and cortex, recognized by tangentially flattened cell layers, and the distinction from cell to cell is not clear in this zone. In some locations of PTM, several vascular bundles diverge into the pith. At the 4-week stage, vascular bundles produced by PTM decrease. The distance of vascular bundle formation increases, and no strands diverge into the pith. PTM cell layers decrease, and endodermis likely form on the inner cortex. At the 6-week stage, the zone between the pith and cortex has 1 – 3 cell layers and no longer produces primary vascular bundles, and lignin endodermis occurs at the inner cortex (Figure 3).
Vascular bundles occurred at 63,33 in the 2-week stage, quickly increased to 94,67 bundles in the 4-week stage, and slowly increased from 94,67 to 106,67 in the 4-week stage and 6-week stages. The cell layers of PTM quickly increased from 1,33 to 6,33 in the 2-week stage, decreased to 3,67 at the 4-week stage and were maintained at the 6-week stage (
The respiratory intensity of the rhizome section decreases from the 0-week stage to the 4-week stage and maintains the 6-week stage. Dry and fresh weight and essential oil drops increased over time (
DISCUSSION
Growth of white turmeric rhizome
In turmeric, during the resting (latent) phase, the stem of the bud has appeared, with a clear distinction between the pillar and the shell, even though the stem is now in the mother rhizome. By definition, tubers in white turmeric are the stem; the latent stage has already appeared, so it is also the stage of tuberous appearance in white turmeric 14. After two weeks of culture, the edematous zone of white turmeric shoots was more pronounced due to increased scaling of parenchyma (Figure 1) and the appearance of essential oils, so the dry weight of the buds also increased (
Activity of the primary thickening meristem in the growth stages of white turmeric rhizomes
White turmeric buds show a “crown structure” with many primordial leaves sheathing the SAM, which is seen in most short internode monocots, such as Arecaceae, Pandanaceae, Musaceae, Agavaceae, and Liliaceae4, 16. There are one or two thin layers of cells between the pith and cortex of the white turmeric bud. It occurs very early in the resting bud stage, beneath the primordial leaves, derives directly from the SAM, and is meristematic but is still not yet active. This cell layer becomes the PTM at later stages, similar to other monocots that have crown bud structures17, 18 (Figure 1, Figure 2, Figure 3).
In the early growth stage, from 0 to 6 weeks under conditions, the radial growth of white turmeric buds occurs as soon as apical growth, finally forming the pear shape of turmeric rhizomes, as Nair (2009) described14. As with other monocots, stem radial thickness is dependent on a special meristem – the primary thickening meristem (PTM), which produces primary vascular bundles19. At the resting bud stage, strands of parenchyma cells derive from the SAM to below between the cortex and pith. These cells discontinuously divide and have a long cell cycle20. According to anatomy results, in white turmeric, after the bud emerges at the 2-week stage, this zone is active to produce primary vascular bundles into the pith and make the pith thick, so the stem grows radially, and the action of PTM strongly produces many primary vascular bundles centripetally. This activity is the same as that of procambium – a pro meristem, which Cheadle (1937) called a thickening ring and De Mason (1983) called a primary thickening meristem (PTM)17, 4. The activity of PTM thickens the cell zone below the SAM, and this radial divide cell activity is the first step in the progress of tuber formation in , potato, and 18, 21, 22. Radial growth by the primary thickening meristem produces primary vascular bundles on one side into the pith and parenchyma cells into the cortex (one side in white turmeric), which is different from the dicot vascular cambium, which produces secondary xylem to the pith and secondary phloem to the cortex20 (Figure 2, Figure 3).
The PTM of white turmeric is active on one side to produce primary vascular bundles to the pith and make the pith thicken, and the cortex still keeps its unchanging size. This activity is the same as ginger on the other hand, similar to , the cortex and pith are thickened by PTM because it is active on two sides, producing primary vascular bundles to the pith and derivatives of the endodermis to the cortex23, 6. The cell layer inner cortex becomes the endodermis, as Menezes . (2005) described: primary thickening in the stems of monocotyledons is due to the meristematic activity of both the endodermis and the pericycle24.
A model hypothesis regarding PTM activity and radial growth in white turmeric
The thickening and the longing apical occur at the same time. The activity of the PTM begins as soon as it grows and depends on the distance between the PTM and SAM. It can be summarized in 4 stages (Figure 4):
- Stage 1, derivation of PTM, PTM derived directly from SAM, has 1 – 2 cell layers and is inactive. Bud has not yet emerged.
- Stage 2, strongly active of PTM, PTM gets a distance from SAM, PTM has 6 – 7 cell layers and acts strongly, and a lot of the primary vascular bundles formed, and rhizome diameter increases strongly too.
- Stage 3 is slowly active in PTM, PTM gets a distance further than stage 2, has 3 – 4 cell layers, is slowly active, decreases the primary vascular bundles formed, and rhizome diameter increases slowly. The inner cortex cell layer prepares to become endodermis.
- Stage 4, inactive PTM, PTM is far from SAM at a distance further than stage 3, there are 2 – 3 cell layers in this area, and endodermis occurs significantly. The rhizomes stop increasing in this stage.
These stages occur at the same time as the prolonged activity the after-growing ring is larger than the before ring, which makes the rhizome a pear shape, or the after ring is the same as the before ring, which makes the rhizome a finger shape.
CONCLUSION
PTM of white turmeric occurred very early between the pith and cortex at the early growing stage (0 – 6 weeks ). PTM white turmeric produced primary vascular bundles into the pith, increasing the size of the pith and the rhizome diameter while maintaining the size of the cortex. PTM was the most strongly active when it included a cell layer that was not clearly distinguished (2-week stage), decreased when the inner cortex cell layer was prepared to become endodermis (4-week stage), and stopped when lignin endodermis occurred (6-week stage).
We recommend continuing the study of the effects of plant hormones on SAM, precursor of endodermis, PTM, STM and accumulation of essential oils in white turmeric.
LIST OF ABBREVIATIONS
PTM: Primary thickening meristem
SAM: Shoot apical meristem
COMPETING INTERESTS
The authors are not conflicts of interest.
AUTHOR CONTRIBUTIONS
Nguyen Hong Buu Vinh performed all experiments. Tran Thi Thanh Hien proposed the idea and wrote the paper. Bui Trang Viet analyzed the results. All authors read and approved the final manuscript.
ACKNOWLEDGEMENT
This research is funded by Viet Nam National University, Ho Chi Minh City (VNU-HCM) under grant number C2022-18-28.

