Determination of ethanol in hand sanitizers by a self-built headspace device and gas chromatography
- Faculty of Chemistry, University of Science, Ho Chi Minh City, 70000 Viet Nam
- Vietnam National University, Ho Chi Minh City, 70000 Viet Nam
Abstract
Introduction: Headspace sampling coupled with gas chromatography (HS-GC) has been proven to be a very useful technique for the determination of volatile and semivolatile components in complex matrices by avoiding tedious sample preparation. In this study, the HS system was fabricated at low cost, exploiting unused GC sensor-heaters.
Methods: The accuracy and stability of the self-built HS were evaluated, and the HS-GC-FID system was applied to the determination of ethanol in hand sanitizer gels. The HS operating conditions, including temperature and equilibrium time, were optimized using a central composite face-centered design (CCFC) and MODDE 5.0 software. Samples incubated at 80 °C for 25 min gave the best results with respect to sensitivity and reproducibility (RSD < 3%).
Results: The calibration curve showed high linearity (R2 > 0.998) in the range of 0.25 to 2.5% (w/w) ethanol, using acetonitrile 1% (w/w) as an internal standard. The recovery ranged between 104.1 and 108.3% using spiked samples on real matrices. A small survey of 11 commercially available samples collected in the local markets showed that only one of them did not meet the standards set by the FDA.
INTRODUCTION
Sample preparation is an essential step to ensure accurate results in chromatographic analysis. It involves extraction of analytes from sample matrices, removal of interferences, and/or concentration of the compounds of interest. The process is quite lengthy and consumes considerable amounts of toxic organic solvents or expensive sorbents. Therefore, it is usually considered a bottleneck step in analytical procedures. Headspace sampling is an effective alternative to liquid‒liquid or solid-phase extraction for volatile and semivolatile analytes.
In the headspace technique, volatile and semivolatile components in solid or liquid samples are kept in closed vials preferably partitioned into the gas phase at high temperatures. After reaching equilibrium, a part of the gas phase will be taken and directly injected into the GC, leaving nonvolatile components in the vials. This results in clean extracts facilitating chromatographic analysis and protecting GC systems from contamination. HS is considered an environmentally friendly sample preparation method because it does not consume any organic solvent. The only thing we have to do is place the raw samples in vials and heat them for a certain period of time. The accuracy and stability of the vial temperature with time are crucial factors that must be well controlled for quantitation purposes and, of course, have to be taken into great consideration when attempting to fabricate one.
In this paper, we present a simple and cost-efficient way to build an HS sampler that can be done by any laboratory where there are GCs with only one detector and injector, which is usually the case. High-quality GC sensor heaters that are usually “forgotten” will be used in this work to control the headspace temperatures.
The performance of our HS device in practice was evaluated by applying it to the determination of ethanol levels in hand sanitizer gels (at least 60% v/v or 52% w/w 1, 2). In the gel form, hand sanitizers contain nonvolatile ingredients, such as polyacrylic acid (carbomer) or xanthan gum, which provides thickness and reduces the evaporation of alcohol3. Samples diluted with water could be injected into GC injectors for ethanol analysis4. However, the nonvolatile components can be problematic in long-term use if they are not removed from the extracts during sample preparation. They deposit gradually in injectors and are carbonized in an inert environment of carrier gas at high temperature, causing a problem well known as the “matrix-induced chromatographic response enhancement effect”. 5
Both the thickeners and ethanol have a high affinity for water, and separating ethanol from the matrices is very difficult. In addition, ethanol is volatile, while polymeric thickeners are not. As a result, it is best suited to HS-GC-FID.
This study consists of two main parts: (i) fabricating an HS sampler and (ii) utilizing the self-built HS to develop an analytical procedure to quantify EtOH levels in hand sanitizer gels. The operating parameters of the HS, namely, equilibrium time and temperature, for this specific application were optimized using an experimental design approach.
MATERIALS – METHODS
Materials and instruments
Ethanol (EtOH) and acetonitrile (ACN) (>99.5%, HPLC grade) were obtained from Fisher Scientific, UK. New bottles of these analytes were used and stored in a desiccator. Glycerol and triethanolamine were products of Xilong Scientific, China, and carbomer 940 was purchased from Corel Pharma Chem, India. Distilled water was used in all experiments.
Ethanol Standard Preparation
Preparation of 2% (v/v) ACN as an internal standard (IS): 2 mL of pure acetonitrile was diluted into a 100 mL volumetric flask with water.
Preparation of 10 % (w/w) EtOH stock solution and working standard solutions: these solutions were prepared by weighing with water first followed by EtOH to minimize EtOH evaporation.
The stock solution was stored in a glass bottle at 4°C and capped tightly. Working standards (0.5, 1.0, 2.0, 4.0, and 5.0% EtOH (w/w)) were prepared daily before use by diluting the EtOH stock solution with distilled water. Then, 1.0 mL of the standard solutions and 1.0 mL of the internal standard were added to the 20 mL HS vials. This results in the final concentrations of EtOH in HS vials being half of the working standards ( 0.25%-2.5%). These vials were closed using a screw cap with a PTFE/silicon septum. The final concentrations of EtOH in HS vials are presented. It should be noted that EtOH standard solutions used to construct the calibration curve were prepared in water only ( without other ingredients).

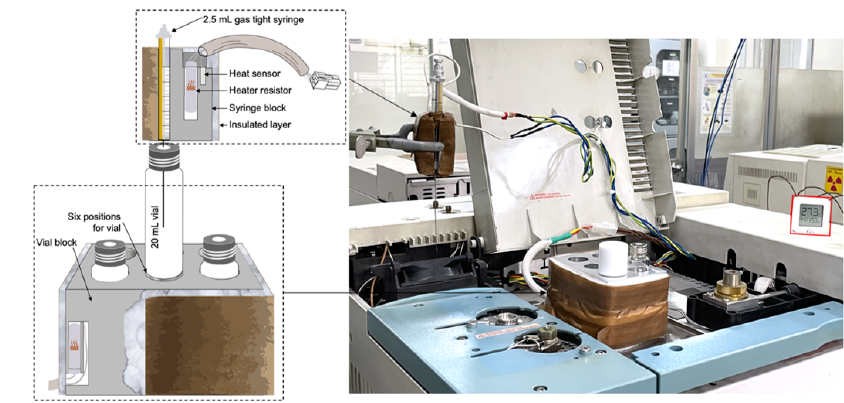
The self-built HS system.
Sample Preparation
Eleven ethanol-based hand sanitizer gels of different brands (marked as 1-11) were randomly purchased from local markets in Ho Chi Minh City, Vietnam, and stored at 4°C. All samples were diluted by weighing instead of withdrawing with pipettes to avoid incomplete delivery because of the high viscosity of the original samples.
Each gel sample was diluted twenty-fold by weighing (10 g of water + 0.5 g of gel sample). Then, 1.0 mL of diluted samples and 1.0 mL of IS solution were placed into HS vials (20 mL). The vials were capped and vortexed to obtain a homogenous solution before analysis. Each sample was prepared in three replicates.
HS fabrication
In 6 heater sensors (G1544-60620) of Agilent GC 6890, there are 2 available; they were employed to control the temperatures of the two metal blocks, one for vials and one for syringes. The heater-sensors were connected and controlled by the auxiliary GC channels.
An aluminum block (20 cm × 10 cm × 10 cm) with six holes (8 cm × 1 cm, height × diameter) was used to thermostat the sample vials. Another aluminum block was used to fit a 2.5 mL gas-tight syringe and had the same operating temperature as the vial block to avoid condensation of samples. A slot was cut vertically along the aluminum block to read the syringe markings.
For insulation, the two metal blocks were covered by a glass wool layer and a layer of insulated tape. Figure 1 illustrates the self-built HS components.
The vial block was placed on top of the GC during operation, as shown in Figure 1. The samples were analyzed using a 6890 N Agilent GC (Agilent Technologies, USA) equipped with a VFWAX column (30 m × 0.32 mm × 0.25 µm). Manual injection (0.5 mL injection volume) was performed with a split ratio of 10:1 at 250 °C, FID was operated at 280°C, and 1.0 mL/min N was used as the carrier gas. The oven temperature was initially set at 45°C, held for 3 min, increased to 60°C (30°C/min), held for 2 min, and finally increased to 150°C (30°C/min), thus giving a total run time of 10.5 min. The temperatures of the vial and syringe metal blocks were set at 80°C. Agilent Chemstation B.04.03.16 software was used for data acquisition and processing.
Experimental Design
The response surface method was applied to obtain optimal HS conditions using a central composited face-centered (CCFC) design and MODDE 5.0 software (Umetrics, Sweden). The effects of two important variables, namely, temperature (X) and equilibration time (X), on the EtOH peak area (Y) in the gas phase were investigated. Based on our scouting experiments, the temperature and equilibrium time ranged from 40 – 80°C and 5 – 35 min, respectively. There were 11 experiments including 3 center points (Figure 2), and the experimental results were fitted to a quadratic model (Eq. 1).

CCFC design to investigate the effects of temperature and equilibration time on EtOH peak area.
Y: response factor
X: independent coded variables
: regression coefficients
RESULTS
The self-built HS system was evaluated with respect to temperature stability and consistency when the ambient temperature changed. In addition, the effect of room temperature on equilibration time was also a part of this investigation.
The temperatures of the vial and syringe blocks, set at 80 °C, were monitored at 3 different room temperatures, 23, 28 and 33°C, using Chemstation software (in the menu).
The data reveal that the two thermostating blocks reached the target temperature after 40 min (Figure 3). The variations in temperature of the syringe and vial blocks were ± 0.1°C and ± 0.05°C, respectively, after equilibrium (Figure 3).

Stability of the syringe and vial blocks set at 80°C at 3 different room temperatures. RT: Room temperature
A CCFC design with 2 variables, namely, equilibration time and temperature, was used to predict the optimal conditions for ethanol determination in hand sanitizer gels. In the design, 11 experiments were conducted; 0.5 mL vapors in the headspace were analyzed by GC-FID, and EtOH peak areas were used as response factors (
The CCFC data were fitted into a quadratic model with variables X and X in coded form (Eq. 2)
Experimental CCFC model results
|
No. |
Factor |
Coded factor |
Y (EtOH peak area) | ||
|
X1 |
X2 |
X1 |
X2 | ||
|
1 |
40 |
5 |
-1 |
-1 |
844.6 |
|
2 |
80 |
5 |
+1 |
-1 |
4988.2 |
|
3 |
40 |
35 |
-1 |
+1 |
860.1 |
|
4 |
80 |
35 |
+1 |
+1 |
5477.3 |
|
5 |
40 |
20 |
-1 |
0 |
1155.5 |
|
6 |
80 |
20 |
+1 |
0 |
5528.6 |
|
7 |
60 |
5 |
0 |
-1 |
2327.0 |
|
8 |
60 |
35 |
0 |
+1 |
2624.9 |
|
9 |
60 |
20 |
0 |
0 |
2928.4 |
|
10 |
60 |
20 |
0 |
0 |
2666.9 |
|
11 |
60 |
20 |
0 |
0 |
2964.4 |
The response surface shows that the highest concentration of EtOH can be obtained at the optimal region where the temperature and equilibration time were 80°C and 25 min, respectively (Figure 4). These conditions will be applied to construct the calibration curve and analyze the real samples.

Response surface showing the effect of temperature and time on the ethanol signal.
The linearity was very good with a high correlation coefficient (R = 0.9982) in the concentration range of 0.25-2.5% EtOH (Figure 5).

The calibration curve of EtOH using HS-GC-FID.

HS-GC-FID chromatogram of a representative hand sanitizer sample.
To assess the performance over time of the developed method using our self-built HS device, a control chart was built by analyzing spiked samples with a concentration of 70% EtOH (w/w) for 21 days. Blank samples as well as spiked samples were prepared in a matrix consisting of 0.3% (w/v) carbomer 940, 1.45% (w/v) glycerol, and triethanolamine to adjust the pH to 6.0.

The control chart for quality control tests.
The EtOH levels in 11 commercial hand sanitizer gels were determined with the developed method. The ethanol contents ranged from 48.2 to 75.2% (w/w) (
Ethanol content in 11 commercial hand gel sanitizer samples by HS-GC-FID
|
Samples No. |
Ethanol (%, w/w) | |
|
|
Average |
%RSD (n=3) |
|
1 |
59.5 |
2.9 |
|
2 |
65.7 |
1.8 |
|
3 |
65.7 |
1.2 |
|
4 |
65.3 |
1.8 |
|
5 |
68 |
2.3 |
|
6 |
69.8 |
2.2 |
|
7 |
75.2 |
0.8 |
|
8 |
73.4 |
2.5 |
|
9 |
48.2 |
1.8 |
|
10 |
70.5 |
2.4 |
|
11 |
61 |
2.2 |
DISCUSSIONS
The results showed that within the normal temperature range of our air-conditioned lab, the ambient temperature had an insignificant effect on their performance (Figure 3).
According to Eq. 2, the temperature (X) had the highest coefficient, implying that temperature is the decisive factor contributing to the EtOH signal (peak area). It is obvious that as the temperature increased, the partition coefficient K decreased. K is the ratio of EtOH concentration in the sample (C) to that in the gas phase (C) at equilibrium. The higher the temperature was, the higher the EtOH concentration in the headspace. In addition, the positive but low regression coefficient of equilibration time (X) indicates that the partition of EtOH from the sample phase to the gas phase required some time to reach equilibrium.
For quantitation using headspace, the concentration of an analyte in the gas phase (C) is directly proportional to its concentration in the original sample (C) if equilibrium is reached (C/C = K) and the phase ratio is kept constant ( = V/V) (Eq. 3 and Eq. 4) 6. Cs and Vs are concentrations in the sample at equilibrium and sample volume, respectively.
In headspace sampling, a calibration curve must be constructed in the same manner as the sample. In other words, the standards must have the same matrix as the sample and undergo headspace sampling before GC analysis. However, to simplify the standard preparation, a small test was conducted to determine whether there was any effect of the matrix, the thickener, or other ingredients on the analytical signal (the ratio of the peak area of EtOH to ACN). Standards with identical EtOH and ACN contents with and without matrix ingredients were analyzed under the same HS and GC conditions. The t test analysis (P > 0.05) showed no significant differences between the analytical signals of standards prepared in distilled water and in the sample matrix. The P value for the t tests evaluated at 60% (w/w) EtOH was 0.55 (data not shown). Therefore, the calibration curve was later constructed using EtOH standards in distilled water only.
One of the advantages of HS sampling is that only volatile components in samples can enter the GC system therefore, the chromatograms of real samples are usually very clean, as shown in Figure 6.
The blank chromatogram of the blank had no signal of EtOH. The recoveries in 21 days showed that there was no value exceeding the action limits (mean ± 3 SD) and 2 successive values exceeding the warning limits (mean ± 2 SD) (Figure 7). In other words, the method is reliable to use.
Comparing our results with those reported previously using the same technique, HS-GC-FID, we found somewhat lower accuracy (6% . 3%) but better precision (0.8 - 2.9% 1.4 - 10.9%) 7, similar to a study using solid-phase microextraction (~2 %)8.
CONCLUSIONS
In this study, an HS device was successfully fabricated at a very low cost, approximately 340 USD, and can be used for the determination of volatile compounds in complex matrices with high repeatability. In addition, a simple and reliable method to determine EtOH concentration in hand sanitizer gels using our self-built headspace device incorporated with GC-FID was developed, validated, and successfully applied to real samples. The clean extracts by headspace allow applications at much lower concentrations of volatile and semivolatile analytes in complex matrices.
Regarding the quality of commercial hand sanitizer gels in local markets, most of them met the international requirements of EtOH levels. That would be very encouraging news for the consumers.
ACKNOWLEDGMENTS
This research is funded by the University of Science, VNU-HCM under grant number T2023-68.
Technical support from Mr. Khue Van Le from Hop Tam Trading – Service Company Limited is highly appreciated.
LIST OF ABBREVIATIONS
COVID-19: Coronavirus disease 2019
FDA: Food and Drug Administration
WHO: World Health Organization
HS-GC-FID: Headspace gas chromatography conducted flame ionization detecter
RSD: Relative standard deviation
IS: Internal standard
CCFC: Central composited face-centered
SD: Standard deviation
UAL: Upper action limit
UWL: Upper warning limit
LWL: Lower warning limit
LAL: Lower Action limit.
Author Contributions
Gia-Huy Hoang Dang carried out the experiment with technical support from Thinh Phuc Nguyen. Gia-Huy Hoang Dang wrote the manuscript in consultation with Mai Anh Nguyen. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest in this study.

