Removing tannins and debittering brewer’s spent yeasts by Tween 80
- The University of Danang, University of Science and Technology, 54 Nguyen Luong Bang, Danang city
Abstract
Introduction: Yeast extract from brewer's spent yeast cells is a promising nutrition source for many applications in the food industry and biomass production. However, because of the impurities that cause the bitter and astringent flavor attributed to hop resins during fermentation, brewer's spent yeasts in the industry have not been widely used. Contaminants have been withdrawn by using a variety of techniques. However, most reported methods have focused on removing iso-alpha acid rather than tannin, which has anti-digestive and antimicrobial properties. This study focuses on removing tannins and evaluating their impact on reusing brewer's spent yeast in biomass production.
Methods: In this work, Tween 80 was employed for the removal of tannins and other bitter substances from brewer’s spent yeasts. In addition to biochemical approaches, the efficiency of cultivating probiotics with autolysates from yeasts cleaned by various buffers is used to evaluate the effectiveness of eliminating tannins and bitter compounds.
Results: In tannin removal, the outstanding efficiency of Tween 80 was four times higher than that of water and hypotonic 0.5% NaCl solution. The debittering efficiency increases from 77% to 83.6% by using Tween 80. Therefore, the cell density of Lactobacillus fermentum 4C cultures with the autolysate from Tween 80-treated spent yeast cells increased by 16.5% and 10.2% compared to that of cultures washed with distilled water or 0.5% NaCl, respectively. The autolysate of Tween 80-treated yeast cells is rich in protein and α-amino nitrogen. It shows advantages in culturing different strains of probiotics.
Conclusions: By skipping the first stage of biomass creation, the reuse of spent yeasts in the production of yeast extracts will not only address environmental issues but also have a positive economic impact.
INTRODUCTION
Beer is a popular drink around the world. Only Heineken makes and exports this Dutch beer to more than 190 countries, with an estimated production of 63.3 mhl (in millions of hectoliters) sold in 2022 1. In Asia Pacific, it produced 12.2 mhl, accounting for 27% of the market share in this region1. Vietnam is a significant market for Heineken, with considerable growth potential. In September 2022, Heineken disclosed its plans to increase production in Vung Tau, the largest brewery in Southeast Asia. In addition, a report by Vietnam Industry Research and Consultancy (VIRAC) states that Vietnamese citizens consume over 4 billion liters of beer annually, ranking third in Asia for beer consumption by 20222. Fifty to fifty-five million yeast cells per gram of dry yeast matter are produced throughout the fermentation process for Heineken beer3. With the current large beer production, although as small as yeast cells, the waste generated is of concern. The brewer's spent yeast is rich in protein, minerals, zinc, chromium, and vitamins (particularly B) [4]. These can provide significant advantages as implemented in medications, human food, and other uses.
After the yeast cells are disrupted, a liquid with soluble components is called yeast extract. The variety of unique physiologically beneficial chemicals found in yeast cells opens up the prospect of utilizing yeast extract in numerous commercial areas4, 5. Yeast extracts are mostly used in the food sector to flavor snacks and to make sauces, gravies, soups, and meat-based products6. Numerous reports have also demonstrated the significance of employing yeast extract in industrial fermentation to produce microbial biomass7.
The reuse of spent yeast to produce yeast extracts will not only solve environmental problems but also bring economic benefits by subtracting the first stage of biomass generation. However, due to impurities during fermentation, such as the bitterness attributed to hop resins 8, brewer's spent yeast has not been used frequently in industry. Several techniques for eliminating impurity chemicals were developed and mostly focused on debittering methods, which refers to the removal of iso-alpha acids, the principal source of bitterness in beer, but not tannin9, 10.
In a technical sense, tannins are polyphenols extracted from plants. Tannins from grape skins enhance the deep tactile texture of wine. The precipitation of complexes and a reduction in in-mouth lubrication are the results of interactions between tannins and salivary proteins, which provide the tactile sensation known as astringency11. Tannins have been shown to be present in grain husks and hops12, aiming at clarifying beer by coagulation of protein, and they are possibly adsorbed on yeast during fermentation13. The presence of tannins improves the integrity of cell walls and protects yeast cells from the effect of ethanol during alcohol biosynthesis. This protection may be due to increased stimulation of -glucan synthesis14 or the prevention of the activity of autolytic enzymes on the yeast cell walls by tannins. However, tannins have been considered digestive inhibitors and bactericides 15, 16. Contaminated tannins can reduce the effectiveness of yeast extract in their application, both in food and microbial biomass production.
Debittering brewer's spent yeast (BSY) is a process aimed at reducing the bitterness of this byproduct to make it more palatable for various applications, such as animal feed or human consumption. Several methods for debittering BSY have been developed. Chemical treatments often involve the use of alkalis (e.g., sodium hydroxide) to adjust the pH of the BSY, which can reduce bitterness. Optimal solubilization of bitter constituents can be achieved by subjecting them to a higher pH level of pH 10 and to heat treatment at a temperature exceeding 50°C. Higher temperatures enhance debittering17. However, excessive use of chemicals can alter the nutritional composition of BSY and result in environmental concerns if not properly managed. The pH adjustment may need to be fine-tuned to avoid overprocessing. In addition, prolonged or excessive heat can cause nutrient losses and undesirable changes in BSY's nutritional profile.
Tween-80 is a polysorbate surfactant with a long polyoxyethylene chain containing a fatty acid ester moiety. It is a mild surfactant that effectively solubilizes solids and removes contaminants. Adding Tween 80 to hypochlorite solution reduced lettuce leaf microbes by 99.6% 18. Tween 80 also significantly enhanced virus removal (3- to 3.6-log reduction in virus titer) from all MNV-1-contaminated fresh produce (strawberries, raspberries, cabbage, and lettuce)19. In addition, Tween 80 exhibited the ability to break the bonds between proteins and tannic acid when added to solutions containing these complexes and thus re-emerges the characteristic spectrum of the tested proteins 20. Tween 80 is considered safe for human consumption and is used as a food additive under the name E433. For instance, it is added to ice cream in amounts up to 0.5% (v/v) to increase the consistency and handleability of the ice cream as well as its resistance to melting21. Therefore, Tween 80 is a promising and safe detergent for removing tannins and other bitter substances from BSY without any negative impact on the quality of the final product.
This study evaluates the impact of tannin removal on the reuse of brewer's spent yeast for biomass production. To do that, Tween 80 has been employed to strip brewer’s spent yeast cells of tannins and other bitter compounds before producing yeast extract. The amount of the protein and α-amino nitrogen was measured to express the nutritional value of the produced yeast extract. In addition, replacing commercial yeast extracts with extracts from spent yeast cells in probiotic cultures also yielded similar and much better cell densities than media without extracts. The results show the potential of using this extract in biomass production.
Materials — Methods
Material and experimental units
Ready-mix media, yeast extract, and other media components for microbial culture were purchased from HiMedia Laboratories, India. Chemicals for preparing washing solutions and for analysis experiments were provided by Xilong Scientific Co., Ltd, China with analytical grade. Otherwise, it will be stated.
Brewer's spent yeast slurry was kindly provided by HEINEKEN Vietnam Brewery Limited Company in Danang city, Vietnam.
Probiotic strains were preserved and provided by the Biotechnology Laboratory, The University of Danang, University of Science and Technology and Cell Laboratory, Institute of Biotechnology, Hue University of Science. They include 4C, sp., and BL21 (DE3).
Methods
Spent yeast slurry collected from the brewery was stored in a sterilized flask. To remove the beer liquor, brewer’s yeast slurry was refrigerated (4-10°C) overnight, and then the liquid at the top was dumped, retaining the sludge on the bottom. Before conducting the experiments, the brewer's spent yeast slurry was examined under a microscope and by culture on nutrient and MRS media to ensure the microbial purity of the yeast waste.
The cream yeast (harvested after giving an off-white liquid) was washed in buffer 1 (distilled water), buffer 2 (0.5% NaCl), and buffer 3 (0.1% Tween 80) at a weight ratio of 1:3 (cream yeast:buffer) before further processing. After gently stirring with a glass rod, the cream yeast was centrifuged at 3500 rpm for 15 mins at 4°C (Hettich Rotina 420 R, Germany). Washing buffers collected from each wash were subjected to tannin and iso-alpha acid determination.
Autolysis was carried out in sterilized glass jars in duplicate. The debittered yeast slurry was resuspended in autolysis buffer (1.5% (v/v) ethyl acetate and 0.086 M NaCl, pH 5.5) and adjusted to an OD value of 40 22. Autolysis was performed at 50°C in an incubator (Daihan Scientific IS-30, South Korea) with a shaking speed of 200 rpm. During autolysis, samples were taken, pasteurized at 80°C for 30 mins to terminate the enzyme activity, cooled to room temperature, and then centrifuged at 3500 rpm for 15 mins at 4°C for chemical analysis of the supernatant at the indicated time points.
Yeast cells at 0 h and 36 h of autolysis were observed under an Olympus optical microscope at 40X magnification of the objective lens.
Dry yeast weight and solid content were determined by drying the condensed solution in an oven (Memmert SF260plus, Germany) at 105°C to a constant weight. The total carbohydrate content was determined by Bertrand’s method 23. The Kjeldahl method was used to analyze the total nitrogen content in dried spent yeast cells. The protein content was estimated by multiplying the total nitrogen content by 6.2524. Samples are burned at high temperatures (500–600°C) in a muffle furnace to assess the amount of ash produced25. Then, the collected ash was dissolved in 3 M HCl solution, and the residual residue was filtered and burnt to determine the amount of acid insoluble ash. In addition, to ensure the results, the collected brewer’s spent yeasts were sent to the testing center TSL (TSL Science Co., Ltd) for analysis and double examination of some important parameters, such as protein, carbohydrate, lipid and calories.
The protein content in yeast extracts was determined by the Biuret method 26 with casein as the standard and a linear curve from 0-50 mg/mL (R is 0.9952). Alpha-amino nitrogen was measured by the ninhydrin method using glutamic acid as a standard and a linear curve from 0.2-0.6 mM (R is 0.9789) 6. The tannin content in the washings and in the yeast extracts was determined by UV‒Vis spectroscopy (Dlab SP-UV1100, China) as described previously27, 28, using tannic acid as a standard and a linear curve from 0.625-10 mg/L (R is 0.9992). The bitter substances in the samples were quantified by the European Brewery Convention (EBC) method9, 29.
The sonication treatments were carried out in continuous mode with the following parameters (Misonix XL-2000, New York, USA): approximately 1.3-1.5 g of dry yeast, 40 ml of D.I. water, ultrasound amplitude of 40%, temperature of 5°C, sonication time of 5 min, probe depth of 1 cm, and 60% of the maximum amplitudes30. The amount of tannin and bitterness units of this sonicated solution were determined as the total amount of these compounds per dry weight of yeast cells.
The yields of protein or α-amino nitrogen content extracted from dry yeast cells were calculated by the following formula:
C = (Amount of protein or α-amino nitrogen)/(weight of dry yeast slurry used) (mg/g or mmol/100 g) (1)
The percentage yield of bitterness removal was calculated by the following formula:
Y = (total EBU in washing solution per dry weight of yeast cells)/(EBU of yeast cells disrupted by sonication per dry weight of yeast cells)*100(%) (2)
The percentage yield of bitterness removal was calculated by the following formula:
Y = (total tannin amount in washing solution per dry weight of yeast cells *100 (%))/(tannin amount of yeast cells disrupted by sonication per dry weight of yeast cells) (3)
Extracellular protease activity and culture of microorganisms
The extracellular crude enzymes were collected by centrifugation of overnight cultures of microbial cells at 6000 rpm for 5 minutes at 4°C. Two hundred microliters of supernatant was placed into the hole in an agar plate containing 2% casein to investigate the microbial extracellular protease. Transparent circles due to casein degradation represented microbial protease activity.
Media for the culture of each probiotic strain were prepared according to the corresponding recipe. 4C was grown in MRS medium supplemented with 1.5% NaCl (10 g/L meat extract; 5 g/L yeast extract; 10 g/L peptone; 20 g/L D-glucose; 8.3 g/L CHCOONa.3HO; 2.62 g/L KHPO.3HO; 0.12 g/L MgSO. HO; 0.06 g/L MnSO. HO; 1% Tween 80; 15 g/L NaCl)31. The growth medium of was YDP (20 g/L peptone; 10 g/L yeast extract; 20 g/L glucose) 32, and BL21 (DE3) was cultured in LB (10 g/L tryptone; 5 g/L yeast extract; 10 g/L NaCl)33. After autolysis, the solution was concentrated at 80-85°C with stirring, and in 6 hours, the concentration of soluble solids was 90.56 g/L. The concentration of produced yeast extract added to growth media was calculated based on the dry weight matter in the extracts. Commercial yeast extract was used as a control.
All strains of bacteria were grown in an incubator at a temperature of 30-37°C with shaking at 140-150 rpm. The change in cell density after 20-22 h of culture in different media is illustrated by optical density at 600 nm. The OD value shown in the graph is 10 times diluted to ensure an OD value within the measured range of the instrument.
The results obtained were evaluated for statistical significance by ANOVA, and Tukey's honest significant difference test or Duncan’s multiple range test was used for statistical analysis of differences in the mean of groups (p<0.05). All statistical analyses were carried out by using the Windows program SPSS version 10.0 (Chicago, IL) and Microsoft Excel 2019. The results are shown as the means and standard deviations obtained from at least three independent experiments.
RESULTS
Characteristics of brewer's spent yeast slurry
Brewer's spent yeast slurry was collected from a local Heineken brewery and stored in a sterilized container for transport to the laboratory. The characteristics of the brewer's yeast slurry were analyzed and are presented in
Characteristics of brewer's spent yeast cells.
|
Characteristics |
Units |
Value |
|
pH |
4.9-5.1 | |
|
Moisture of slurry |
% |
80.7 - 90.35 |
|
Calories |
kcal/100 g |
364 |
|
Protein |
wt% dry yeast cells |
38.3 - 40.2 |
|
Total sugar |
wt% dry yeast cells |
49.2 - 50.7 |
|
Total lipid |
wt% dry yeast cells |
0.908 – 1.18 |
|
Total ash |
wt% dry yeast cells |
5.034-5.036 |
|
Acid insoluble ash |
wt% dry yeast cells |
0.037-0.038 |
The excessive moisture in the yeast slurry of approximately 90% has been a major obstacle in the reuse of yeast extracts due to the large volume required for storage. However, these yeast cells settle down at low temperatures. Therefore, the appropriate settling time at refrigerator temperature (4 - 10°C) to remove excessive liquor before performing further experiments was determined. The results showed that after 12 h of sedimentation, most of the spent yeasts settled and stuck quite firmly to the bottom of the container, and then the supernatant was taken out conveniently. The remaining yeast slurry had approximately 60% moisture.
The analysis of spent yeast composition showed that this was a rich source of protein (38.3-40.2% dry yeasts) and carbohydrate (49.2-50.7% dry yeasts), and thus, this has been a valuable source for many different applications (
Tween 80 for decontaminating brewer’s spent yeast cells
Tannins and other bitter substances from hop resins are the main contaminants causing the bitter and astringent flavor 34 in the extract produced from brewers’ spent yeast slurry. Moreover, tannin and iso-alpha acid possess antibacterial activity35, 36, which is able to inhibit the growth of bacteria cultured in media containing produced yeast extract from them.

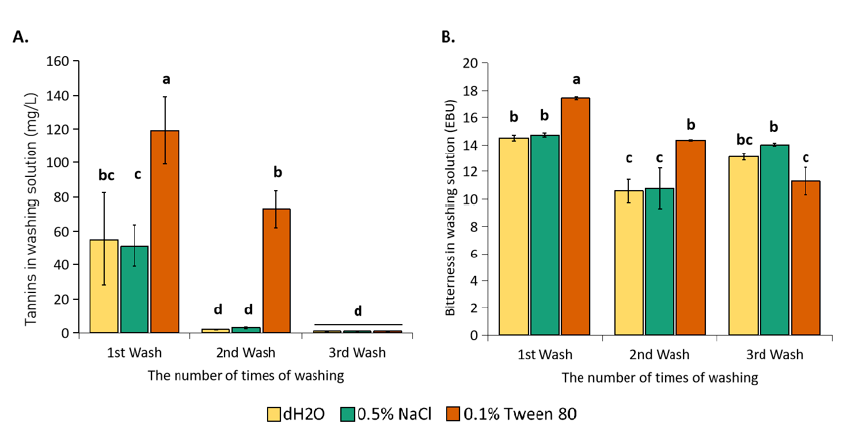
The amount of removal tannin (A.) and bitter substances (B.) from spent yeasts by different wash buffers. The results were obtained by three independent experiments. Means and standard deviation are presented. Means not sharing alphabetic characters differ significantly at p<0.05 as indicated by Turkey’s HSD.
To remove impurities, three different types of wash buffers were employed in this study, including distilled water, 0.5% NaCl, and 0.1% Tween 80 solution. Compared with other wash buffers, the removal efficiency of tannin and bitter substances by Tween 80 was the most effective, except in the third wash to remove bitter substances. Especially for tannins, using Tween 80 gave significantly superior washing power with double as much amount in the 1 wash compared to the other wash solutions, and in the 2 wash, the tannin was removed by only Tween 80 (Figure 1A). After two washings with 0.1% Tween 80, the tannin removal effectiveness was 93.2% as opposed to the 25% of tannins removed by distilled water and 0.5% NaCl. The results also showed that the tannin content in the wash solution varied considerably in different batches (Figure 1A), probably due to the fluctuations of contaminated tannins. Tannins are not a strictly controlled indicator in brewing, and their amount depends on hop quality and barley bark. Meanwhile, bitterness is an important criterion of beer quality, so its content was relatively stable, and the variability was quite low (Figure 1B). The yield of bitterness removal after washing with distilled water, hypotonic 0.5% NaCl and 0.1% Tween 80 solution was 77.7%, 79.65%, and 83.6%, respectively. The total amount of bitterness removed after three washes was not statistically significant among the three washing solutions. However, in the 1 and 2 washes alone, washing with Tween 80 still prevailed, and thus, using a mild and safe detergent such as Tween 80 saves time in cleaning yeast cells for further applications. Together, these results showed that washing yeast slurry twice is adequate to remove unwanted contaminants from the spent yeast cells, where the tannin content was almost undetectable, and the bitterness content decreased significantly.
Autolysis is a process that occurs when yeast cells die at temperatures above 45°C. This process is caused by a series of autologous enzymes that breakdown the yeast cell wall and membrane, releasing the substances inside the cell into the solution, known as yeast extract. Figure 2 displays yeast cell wall cleavage after 36 hours of autolysis at 50°C. Under the microscope, it was observed that yeast cells no longer had clear borders but instead appeared rough with holes. (Figure 2B).

The microscopy images of spent yeast cells at 0 h (A.) and 36 h (B.) of autolysis at magnification of the objective lens at 40X.
To reassess the removal efficiency of tannin and bitter substances by different wash buffers, the content of these two substances in the autolysates of yeast cells washed by different buffers was measured. The results shown in Figure 3 were compatible with the washing efficiency by Tween 80 observed in Figure 1. The tannins and bitterness units in the autolysates of yeast cells washed with Tween 80 were significantly lower than those in other extracts (Figure 3A&B). Next, the growth of 4C in a medium supplemented with autolysates from yeast cells that had been washed with various buffers was observed 4C is a gram-positive bacterium sensitive to inhibition by tannins and iso-alpha acids37, 38. The sensitivity was verified as the cell biomass of 4C reached its peak when the medium was supplemented with extracts from yeast cells washed with Tween 80 (as shown in Figure 3D), even though there was no difference in protein and α-amino nitrogen content among the extracts (Figure 3C). The 4C density increased by 16.5% and 10.2% with Tween 80-pretreated extract compared to that washed with distilled water or 0.5% NaCl, respectively.
The residual tannins in the autolysate of yeast cells washed with NaCl solution were significantly lower, only approximately 50% of those washed with distilled water (Figure 3A). This observation contradicted the findings in Figure 1A, in which tannins washed out by 0.5% NaCl solution were substantially lower than those washed by 0.1% Tween 80 but on par with those washed with distilled water. Yeast cells washed with 0.5% NaCl and distilled water were thus predicted to have the same tannin content in their autolysate. The reason for this unexpected result is probably due to Na+ strengthening the connections between tannins and proteins or carbohydrates 39, so it is challenging to remove tannins adhering to yeast cell walls or cell membranes during the subsequent washing and autolysis process. Next, the tannin complexes and cell debris are probably precipitated from the autolysis fluid through centrifugation and discarded, so the tannin in the extract was low.

The effect of washing buffers on the yeast extract. After washing by different types of buffers, yeast cells were used for autolyzing to produce yeast extracts. The amount of tannin (A.), bitterness (B.), protein and α-amino nitrogen (C.) in yeast extracts were determined. The efficiency in culturing
The effect of time on autolysis efficiency
The economic efficiency of the production of extracts from spent yeast cells is affected by autolysis time. Next, the change in soluble solids concentration, protein, and α-amino nitrogen content during autolysis of brewer's spent yeasts washed with 0.1% Tween 80 was evaluated.

The change of substance content in the yeast extract during autolysis time. The decrease in dry yeast mass (blue line, closed circle) corresponding to the increase in soluble dry matter concentration (red line, open square) during autolysis time is shown in figure A. Protein concentration (light blue) and α-amino nitrogen (orange) in the autolysis solution as shown in Figure B.
After 6 hours of autolysis, the concentration of soluble solids and protein content within the autolysate increased considerably. Concurrently, there was a significant reduction in the mass of yeast cells (Figure 4). Then, the yeast cell mass and soluble solids content barely changed (Figure 4A). Protein content markedly dropped after 12 hours of autolysis. This is because protease secreted from disintegrated yeast cells degrades protein and causes the concentration of α-amino nitrogen to be enriched (Figure 4B).
MRS (for ) and YPD (for ) media were prepared according to the reported recipes but replaced with yeast extracts at different collection times instead of commercial ones. The media were then used for the cultures of respective probiotics. Here, two strains of probiotics 4C and sp. possessing different abilities to secrete extracellular protease were utilized. In particular, the extracellular protease of was very low compared to as evaluated by casein degradation (Figure 5A&B). Casein breakdown by 4C resulted in a substantially larger translucent circle (27.5 mm) than that by (3.5 mm). As a result, the demand for amino acids or hydrolyzed proteins in the culture medium of the two strains may also be different. The density of showed almost no difference after 20 h of culture in different media, except for the control (Figure 5A). The cells that were grown in the medium containing the extract collected at 12 hours or earlier had a slower growth rate compared to those grown in the medium with yeast extract autolyzed for 24 hours or more, which showed a 10% higher yeast biomass ( < 0.01). It is likely that the lower activity of extracellular proteases in contributes to this phenomenon. To facilitate nutrient absorption and growth, it may be advantageous to add partially hydrolyzed protein or amino acids from extracts during longer autolysis times.

The probiotic culture in medium supplemented with yeast extracts collected at different time points from 0 to 48 hours. The transparent circle shows the hydrolysis of casein by different probiotic strains (A&B) after 24 h of incubation with microbial culture supernatant. The diameter of casein degradation circles - agar holes are shown in figure A&B. Liquid sterile growth media were used as a negative control. Yeast extracts collected at different times were added to the growth medium of probiotic strains. After 20 h of culture with shaking, the probiotic cultures were measured for optical density at 600 nm. YE: yeast extract. **, p value<0.01
The combined findings demonstrated that the extract obtained following a 24-hour autolysis process was suitable for the culture of a variety of probiotic strains with a range of extracellular protease activities. Therefore, in subsequent investigations, the composition and efficacy of the microbial culture were compared to those of commercial yeast extracts by using brewer’s spent yeast extract obtained after 24 hours of autolysis.
Probiotic culture by extract supplementation.
Through the growth of (DE3) in suitable media with the addition of brewer's spent yeast extract, the ability to utilize the extracts for microbial culture was assessed. As a control, microorganisms were grown in media without or with commercial yeast extracts. The amount of yeast extract in the culture medium was adjusted to match the commercial product based on dry matter content.

The density of probiotics cultured in media supplemented with the extract from brewer’s spent yeasts (surplus YE) or with the one from commercial. YE: yeast extract. The medium without YE is used as control.
The addition of yeast extract had a favorable effect on the proliferation of microbial cells (Figure 6). Particularly for BL21 (DE3), which is frequently employed in the manufacture of recombinant proteins, bacterial biomass increased by 300% compared to the medium without yeast extract. The biomass of and also increased by 34.5% and 67.7%, respectively, by the addition of yeast extract. For all the examined microorganism strains, the density of microbial cells that reproduced in the medium supplemented with an extract from brewers' spent yeast cells was equivalent to that in the medium supplied with commercial yeast extract.
DISCUSSION
Although hops contain tannins, barley husks account for most of these tannins in beer production. Tannins in beer tend to decrease throughout fermentation to between 150 and 330 mg of tannins per liter of beer because of their attachment to yeast cells40. Using spent yeast cells for producing yeast extracts may be a disadvantage in industrial applications due to the presence of tannins. Tannins cause astringent taste in foods and inhibit digestive enzyme activity41, 42 and the growth of probiotics such as spp. 43. Regarding tannin adsorption, the interaction with yeasts has primarily been linked to cell walls44 and, more specifically, to mannoproteins, glycoproteins localized outermost layer of the yeast cell wall13. Moreover, recently, it has been postulated that proanthocyanidins (PAs), also known as condensed tannins, could pass through the wall pores and might interact with the plasma membrane 45. Thus, this makes tannin removal from brewer’s spent yeasts more difficult. Our study is the first work dealing with tannin removal from brewer's spent yeasts and illustrating the influence of tannins in the yeast extract on the culture of probiotics (Figure 1, Figure 3). Tannin in the yeast extract had a negative effect on the development of probiotics. Lower growth of 4C was observed in the culture supplemented with the extract containing a higher concentration of tannin from spent yeasts washed with distilled water and NaCl 0.5% solution. Because Tween 80 was more effective at removing tannins, there was less tannin left in the extract, which increased the density of 4C (Figure 3A&D).
Tween 80 (polysorbate 80, polyoxyethylene sorbitan monooleate) is a hydrophilic nonionic surfactant that is frequently employed in the emulsification and dispersion of ingredients in pharmaceutical and food applications46, 47. The number of enzymes excreted increased by 70% — 250% when Tween 80 was added to a medium designed for recombinant fungi or yeast to produce secreted enzymes at a concentration of 0.1% 48, 49. This effect may be related to the fatty acid interference of Tween 80 to increase the cell membrane permeability 50 and thus facilitate the release of intracellular or transmembrane substances into the extracellular fluid. Tween 80 was used at a concentration of 0.2% to 20% v/v, adjusting the pH to 10.0 with 2 NaOH51 for a maximal value of 93% bitterness removal from spent (Ale) and (Lager), but the removal of tannins was not addressed. Although our study with a lower Tween 80 concentration of 0.1% at neutral pH obtained a lower efficiency of bitter removal, approximately 83.6% (Figure 2), many advantages should be considered. One of them is a lesser number of washes because no more rinsing is required to remove NaOH and excessive Tween 80. In addition, at neutral pH, the color of the extract is much lighter than that of the extract made from spent yeasts washed at high pH, which may have a negative effect on the color of the product later. Moreover, to replace toxic alkali solutions for debittering, rotary microfiltration as a means to combine debittering and cell debris separation into a single step was carried out with the highest bitter removal efficiency of 86% 9. Although our cleaning method was almost equally effective (83.6%), it is simpler and has lower initial investment costs for equipment.
In addition, the obtained extract had a relatively similar protein and α-amino nitrogen content to those of previous studies52, and the protein recovery rate was over 95%. This indicates that Tween 80 does not damage the yeast cell wall or inhibit the activity of protease enzymes that are released from the cell and thus avoids nutrient loss during washing.
CONCLUSIONS
It has been determined that the 0.1% Tween 80 solution works best for eliminating tannins and other bitter compounds from brewer’s spent yeast cells, thereby lessening their concentration in the extract. The resulting yeast extract is ideal for the growth of numerous different probiotic strains. During autolysis, the extract had opposite protein and amino nitrogen concentrations; the protein concentration dropped while the amino nitrogen concentration rose in correlation with the activity of the protease enzyme. After 24 hours, the extract produced levels of protein and α-amino nitrogen appropriate for the cultivation of various bacterial species with varying degrees of extracellular protease activity.
ABBREVIATIONS
BSY: brewer's spent yeast
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGEMENT
This work was supported by The University of Danang, University of Science and Technology, code number of Project: T2022-02-38.
AUTHOR CONTRIBUTIONS
xxx

