Synthesis of donor and acceptor monomers for conjugated polymers in organic optoelectronic applications
- National Key Laboratory of Polymer and Composite Materials
- Faculty of Chemical Engineering, Ho Chi Minh City University of Technology
- National Key Laboratory of Polymer and Composite Materials, Ho Chi Minh City University of Technology, Vietnam National University (VNU-HCM), 268 Ly Thuong Kiet, District 10, 70000 Ho Chi Minh City, Vietnam
Abstract
Introduction: In today's life, conductive polymers play an increasingly important role, and it is very important to research and find materials with new properties and the ability to improve the performance of devices. The synthesis of monomer units as donor-acceptor structural units in conducting polymers is extremely important.
Methods: In this research, the process of synthesizing these potential monomer structural units with high efficiency has been described, including three main units, (4-hexyl-phenyl)(10H-phenoxazin-10-yl)methanone (HP-POzM) (58%, yield), 4,8-bis(hexyloxy)benzo[1,2-b:4,5-b']dithiophene (BHBDT) (90%, yield) and 5-(2-ethylhexyl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione (EHTPD) (95%, yield).
Results: The successful synthesis of monomers was demonstrated by qualitative analytical measurements of 1H NMR and TLC monitor. In addition, it is an overview of the direction that scientists are aiming for these monomer units, as D-A units in conductive polymers, applied in current fields and development orientation in the future.
INTRODUCTION
Phenoxazine (POZ) contains oxygen and nitrogen elements in heterocyclic compounds that are high electron donors, so this phenoxazine acts as an electron donor. Compared to donor compounds such as carbazole and thiophene, the electron-rich oxygen atom in phenoxazine can be a very high electron donor because the ionization potential is lower than 0.7 eV and it is a stable cationic radical. The nonplanar structure of phenoxazine can hinder intermolecular p-stacking and excimer formation.1, 2, 3, 4, 5 In addition, most fluorescent molecules based on phenoxazine can emit blue and red light. Therefore, phenoxazine and its derivative have potential in organic solar cell (OSC) and organic light emitting diode (OLED) applications.
In recent years, the electron mobility of phenoxazine derivatives has been greatly improved, allowing organic field effect transistors (OFETs) based on phenoxazine to achieve high operating efficiency. To achieve efficient OFETs, charge carriers must be transmitted through the semiconductor channel with the lowest “trapped” state. To minimize these "traps", the arrangement of the organic semiconductors is very important. The presence of unsaturated and heterocyclic rings in phenoxazine increases hole mobility through this material, so POZ has great potential when combined with other structural units to form a p-type semiconductor in OFETs. 6, 7, 8, 9, 10 In addition, (4-hexylphenyl)(10H-phenoxazin-10-yl)methanone (HP-POzM) modified from POZ incorporated with a benzyl hexyl side chain increased the solubility and self-assembly of the formed conjugated polymers.
On the other hand, benzo[1,2-b:4,5-b' ]dithiophene (BDT) has a planar and symmetric conjugated structure, and thus, it can stack tightly and evenly for conjugated polymers based on BDT. Therefore, BDT-based polymers were first used in organic field transistors (OFETs). In 2007, a BDT-thiophene-based polymer was reported and exhibited a hole mobility of 0.25 cmVs, which is among the highest values of polymer-based OFETs.11 In 2008, Hou et al introduced a BDT-based conjugated polymer for the first time in solar cells as synthesized BDT-based photovoltaic polymers. The energy level of the BDT-based polymer has been successfully tuned by the molecular structure.12 In addition, the BDT-thiophene-based polymer shows wide absorption and low band gap energy, which are promising for photovoltaic applications. Other research groups further reported photovoltaic materials based on BDT. Copolymers including BDT units and thiophene [3,4-b] thiophene units have high photovoltaic efficiencies of 5%–7%,13, 14 and the results indicate that BDT can be a useful unit in the construction of conjugated polymers as a new donor for high-performance PSCs. Therefore, BDT has great potential in OSC and OFET applications. Moreover, the 4,8-bis(hexyloxy)benzo[1,2-b:4,5-b']dithiophene (BHBDT) monomer that was installed on the hexyl side in its structure increased the solubility of conjugated polymers, which led to charge transfer in optoelectronic layers.
Thieno [3,4-c] pyrrole-4,6-dione (TPD) is an attractive and potentially rich unit because of its compact planar structure. The electrons in the TPD moieties can be delocalized if they are incorporated into other moieties in conjugated polymers.15 Furthermore, it has strong electron-withdrawing properties that result in lower HOMO and LUMO energy levels, a desirable property that will increase stability and V in BHJ solar cells. Furthermore, TPD can be easily synthesized in a few steps based on commercially available compounds. Several research groups have synthesized copolymers based on TPD units that can achieve high energy conversion efficiency.12, 14, 16, 17, 18, 19, 20, 21 The 5-(2-ethylhexyl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione (EHTPD) monomer was based on the TPD monomer, which was also a strong acceptor unit; however, EHTPD has a 5-(2-ethylhexyl) side chain that will also increase the solubility of conjugated polymers containing EHTPD moieties.
Based on the potentials of monomers, including POZ, TPD and BDT, which have been mentioned above, in this research, the derivatives of POZ, TPD and BDT monomers, including HP-POzM, BHBDT and EHTPD, will be synthesized and characterized. These monomers will increase their solubility in common solvents for advanced solution processing, which makes the efficient charge transfer of thin films possible. These novel monomers will be promising synthesized units for new conjugated polymers that can be applied in organic solar cells and sensory applications.
EXPERIMENTAL
Materials
4-(4-hexylphenyl)-4H-dithieno[3,2-B:2’,3’d]pyrrole (98%), 3-hexylthiophene (99%), 10H-phenoxazine (98%, Acros), 3-(bromomethyl)heptane, 2-ethyhexyl bromide (98%), 4-hexylbenzoyl chloride (98%), benzo[1,2-b:4,5-b']dithiophene-4,8-dione, 1-bromohexane, -bromosuccinimide (NBS, Acros), thiophene-3,4-dicarboxylic acid, acetic anhydride, 2-ethyl-1-hexylamine and thionyl chloride were purchased from Acros Organic and used as received.
Chloroform (CHCl, 99.5%), tetrahydrofurane (THF) and dichloromethane (CHCl) were ordered from Fishe and stored under N. Methanol (99%) and acetonitrile (CHCN, 99%) were ordered from Fisher and used as received.
Triethyl amine (TEA, 99%), NaOt-Bu (99%), KCO (99%), zinc (Zn, 99%) and tetrabutylammonium bromide (TBAB, Merck) were obtained from Merck.
Characterization
UV–vis absorption spectra of the compound in solution were recorded on an aligent 8453 UV‒Vis spectrometer over a wavelength range of 190 nm – 1100 nm. The concentration of monomers was established as 105 M for UV –Vis measurement. H NMR spectra were recorded in deuterated chloroform (CDCl) with tetramethylsilane as an internal reference on a Bruker Advance 500 MHz. The wavelengths of the TLC testing UV lamp were 254 nm and 365 nm.
Synthesis of (4-hexylphenyl)(10H-phenoxazin-10-yl)methanone (HP-POzM) monomer
The round bottom flask was charged with nitrogen, and the flask was connected to a reflux condenser. 4-hexylbenzoyl chloride (587. mg, 2.62 mmol), phenoxazine (400 mg, 2.18 mmol), 10 mL THF, triethylamine (0.3 mL, 2.18 mmol), and 4-dimethylaminopyridine (45 mg, 0.36 mmol) were added into the flask reaction. Then, the reaction was heated for 72 h. Then, the reaction was cooled, and the solvent was evaporated under vacuum evaporation. The mixture was mixed with 30 ml of distilled water, and the solution was extracted with dichloromethane. The recovered organic layer was evaporated, and the residue was purified by chromatography using silica gel with hexane/ethyl acetate:15/1 as the eluent solvent. The colorless oil (512 mg, 75%) was obtained as the pure product of HP-POzM. The purity degree of the product was established by H NMR: 99%.
Synthesis of 4,8-bis(hexyloxy)benzo[1,2-b:4,5-b']dithiophene (BHBDT) monomer
Monomer BHBDT was synthesized from benzo[1,2-b:4,5-b']dithiophene-4,8-dione and 1-bromohexane with a molar ratio of 1:3 under nitrogene conditions. A Schlenk tube was charged with benzo[1,2-b:4,5-b']dithiophene-4,8-dione (220 mg, 1 mmol) and zinc (145 mg) in a solution of 12% NaOH. The mixture was stirred for 2 h, and then tetrabutylammonium bromide (TBAB) (64 mg, 0.2 mmol) catalyst and 1-bromohexane (495 mg, 3 mmol) were added into the solution. The reaction was performed at room temperature for 24 hours. When the reaction was finished, the mixture was evaporated to remove the solvent. Then, 30 mL of water was added to the mixture and extracted with dichloromethane. After that, the residue mixture was collected by rotary evaporation. The mixture was purified by chromatography (silica gel, dichloromethane) to obtain the purity of the BHBDT monomer. The product was dried under vacuum at 50 °C for 24 h (320 mg, 90%). The purity degree of the product was established by H NMR: 99.5%.
Synthesis of 5-(2-ethylhexyl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione (EHTPD) monomer
2-Ethyl-1-hexylamine (712 mg, 4.01 mmol), thieno[3,4-c]furan-1,3-dione (1000 mg, 3.09 mmol) and thionyl chloride (1187 mg, 12.34 mmol) were added to a 100 mL two-necked round bottom flask under nitrogen flow. Then, dry toluene was added to dissolve the compounds. The solution was degassed via a freeze–pump–thaw cycle three times. Then, the reaction was heated at 50 °C for 24 h, after which the solution was cooled to room temperature. Then, 50 mL of dichloromethane was added to extract the organic solvent, which was dried over anhydrous KCO and the solvent was removed by evaporation. The residue was purified over SiO using dichloromethane/n-hexane (1:10, v/v) as an eluent to obtain a pure compound as a white solid (1048 mg, 91%). The purity degree of the product was established by H NMR: 99.5%.
RESULTS
The synthesis of conjugated monomers is presented in Scheme 1. All reactions were performed under nitrogen conditions using borate glassware. The equipment was washed with acetone and dried in an oven for 24 hours. The Schlenk line vacuum was checked by piari gauge measurement to ensure a vacuum value of approximately 10 torr for the experimental conditions.

Synthesis of conjugated monomers, including HP-POzM, BHBDT and EHTPD.
Synthesis and characterization of the HP-POzM monomer.
The HP-POzM monomer was synthesized from commercially available H-phenoxazine with 4-hexylbenzoyl chloride. The H-phenoxazine reacted with 4-hexylbenzoyl chloride via esterification between the NH group and thionyl chloride. Figure 3 presents the mechanism of synthesis of the HP-POzM monomer. The reaction was performed with a reflux condenser. The mixture reaction was checked by TLC plates to determine the formation of the product. The eluent solvent of ethyl acetate and hexane with a ratio volume of 1:50 was used to separate the mixture after the reaction. Figure 2(a) shows the mixture reaction before and after the reaction, and Figure 2(b) presents the TLC template that displays the product and starting materials.

Synthesis of HP-POzM monomer (a) left (before reaction); right (after reaction). TLC template of starting materials and products (b).

The mechanism of synthesis of the HP-POzM monomer.
Furthermore, the reaction mixture was purified to collect the pure compound for H NMR analysis. Figure 4 presents the NMR spectrum of the HP-POzM monomer.

1H NMR spectrum of the HP-POzM monomer.
Synthesis and characterization of the BHBDT monomer.
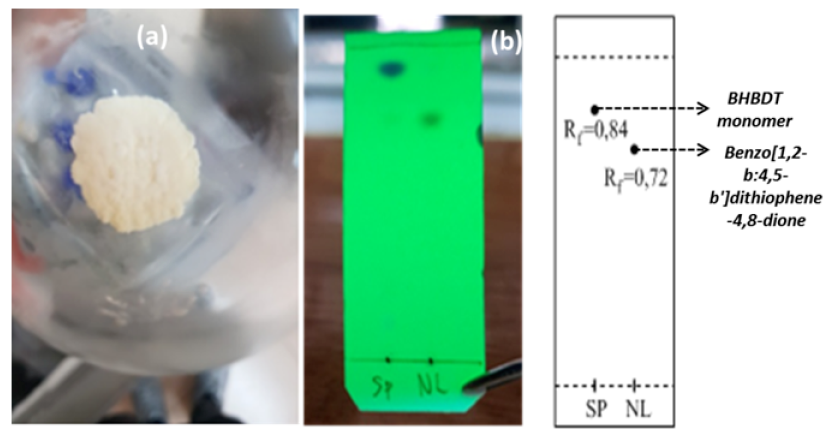
The BHBDT monomer was synthesized from benzo[1,2-b:4,5-b']dithiophene-4,8-dione and 1-bromohexane at a molar ratio of 1:3. The reaction followed the nucleophile mechasim, where Zn and TBAB were used as catalyst systems. Figure 6 presents the mechanism of synthesis of the BHBDT monomer. The reaction was performed at room temperature for 24 h. The reaction was monitored by TLC using dichloromethane (DCM) as the eluent for the chromatography column. The product of the reaction is a white solid powder that is attributed to the pure product of the BHBDT monomer. Figure 5 shows a white solid powder of monomer and TLC results.

BHBDT monomer as a white solid powder (a). TLC template of starting materials and BHBDT monomer (b).

The mechanism of synthesis of the BHBDT monomer.
The BHBDT monomer was dried under vacuum at 50 °C for 24 h and then its chemical structure was characterized via H NMR spectroscopy. Figure 7 shows the H NMR spectrum of the BHBDT monomer.

1H NMR spectrum of the BHBDT monomer.
Synthesis and characterization of the EHTPD monomer
The EHTPD monomer was synthesized from starting materials of 3,3'-dibromo-2,2'-bithiophene with 4-hexylaniline. The reaction was performed via two steps, including the synthesis of the thieno[3,4-c]furan-1,3-dione compound and then the EHTPD monomer. Initially, thiophene-3,4-dicarboxylic acid reacted with acetic anhydride at 140 °C for 12 h to form the intermediate product of thieno[3,4-c]furan-1,3-dione. Then, thieno[3,4-c]furan-1,3-dione was collected and stored in nitrogen for the next reaction. Next, the thieno[3,4-c]furan-1,3-dione will be used to react with 2-ethyl-1-hexylamine in the presence of thionyl chloride (SOCl) to form the desired EHTPD monomer. The reaction was followed with the nucleophile mechasim, and mechasim is presented in Figure 9. It should be noted that this reaction is a volatile reaction because of thionyl chloride therefore, a trap was installed to collect the acid. Figure 8 shows the white crytalline product of the EHTPD monomer and its TLC result.

EHTPD monomer as crystalline white solid powder (a). TLC template of EHTPD monome (b).

The mechanism of synthesis of the EHTPD monomer.
In addition, the EHTPD monomer was purified via acetate/hexane (1:8) as the eluent of the chromatography column. Figure 10 shows the H NMR spectrum of the EHTPD monomer in deuterated chloroform.

1H NMR spectrum of the EHTPD monomer.
DISCUSSION
As seen in Figure 2, it is clear that the R of HP-POzM is 0.86, which is different from that of phenoxazine at 0.69 and 4-hexylbenzoyl chloride at 0.9. This result suggested that the new product was formed in the reaction. The chemical structure of the HP-POzM monomer was characterized via H NMR. In Figure 4, the peaks at 0.87 ppm, 1.34 ppm and 1.59 ppm are attributed to the aliphatic protons of the hexyl side chain. The peak at 2.58 ppm was assigned to the methylene proton on the hexyl side chain. The peaks from 6.93 ppm to 7.4 ppm correspond to the aromatic protons in phenooxazine and the benzene ring. Based on the integration of peaks, we also confirmed that the molecular structure was determined for the HP-POzM monomer.
1H NMR characterization of the HP-POzM monomer.
|
Proton |
Shift δ (ppm) |
Peak |
Number of protons |
Integration of peak |
|
a,b |
7.36 |
Single |
4 |
4.02 |
|
c |
7.10 |
Multiple |
6 |
6.06 |
|
d |
6.93 |
Multiple |
2 |
2.00 |
|
e |
2.58 |
Triplet |
2 |
2.03 |
|
f |
1.59 |
Multiple |
2 |
2.07 |
|
g |
1.34 |
Multiple |
6 |
6.03 |
|
h |
0.87 |
Multiple |
3 |
3.03 |
In the case of the synthesis of the 4,8-bis(hexyloxy)benzo[1,2-b:4,5-b']dithiophene (BHBDT) monomer, the R of the monomer is 0.84, while the R of benzo[1,2-b:4,5-b']dithiophene-4,8-dione is 0.72 (Figure 5). This result suggests that a new monomer compound was formed. The monomer BHBDT was also analyzed by H NMR in deuterated chloroform (Figure 7). In Figure 5, the peaks at 0.92 ppm, 1.45 ppm, 1.56 ppm, 1.6 ppm and 1.87 ppm correspond to the aliphatic protons of the hexyl side chain of the monomer. The peak at 4.28 ppm (peak “c”), which is attributed to the methylene proton, is adjacent to an ether linker. Moreover, the peak at 7.47 ppm is assigned to the proton at position “2” on the thiophene ring, and the peak at 7.35 ppm corresponds to the proton at position “3” of the thiophene ring. In addition, the integration of peaks is reasonable in its chemical structure therefore, 4,8-bis(hexyloxy)benzo[1,2-b:4,5-b']dithiophene has been synthesized successfully by nucleophile reaction. Table 2 shows the 1H NMR results for the BHBDT monomer.
1H NMR characterization of the BHBDT monomer.
|
Proton |
Shift δ (ppm) |
Peak |
Number of protons |
Integration of peak |
|
a |
7.47 |
Double |
2 |
2.00 |
|
7.35 |
Double |
2 |
2.00 | |
|
c |
4.28 |
Triplet |
4 |
4.09 |
|
d |
1.87 |
Multiple |
4 |
4.07 |
|
e |
1.45 |
Multiple |
12 |
12.04 |
|
f |
0.92 |
Multiple |
6 |
61.3 |
According to Figure 8, TLC of the reaction was monitored following the reaction. The R of the 5-(2-ethylhexyl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione (EHTPD) monomer is 0.24, which is compared to the R of the reactant thieno[3,4-c]furan-1,3-dione with an R of zero. This result also suggested that the new compound was formed in the reaction. In Figure 10, the aliphatic protons, including peaks e, f, g, h, i, and k, were observed at 0.89 ppm, 1.30 ppm and 1.6 ppm, respectively. The peak at 1.56 ppm is contamination of water in the analyzed sample. The peak at 3.51 ppm is attributed to the methylene proton on the side alkyl chain adjacent to the nitrogen atom. The peaks at 7.8 ppm correspond to the protons of the thiophene ring. The integration of peaks in H NMR also determined the chemical structure of the EHTPD monomer. Thus, we assure that the EHTPD monomer has been synthesized successfully. Table 3 presents the H NMR results of the EHTPD monomer.
1H NMR characterization of the EHTPD monomer.
|
Proton |
Shift δ (ppm) |
Peak |
Number of protons |
Integration of peak |
|
a, b |
7.80 |
Single |
2 |
2 |
|
c |
3.51 |
Double |
2 |
2 |
|
d |
1.80 |
Multiple |
1 |
1 |
|
e, f, g, h |
1.3 – 1.6 |
Multiple |
8 |
8 |
|
i, k |
0.89 |
Multiple |
6 |
6 |
Finally, three monomers, HP-POzM, BHBDT and EHTPD, were characterized for their absorption properties by UV‒Vis spectroscopy in chloroform. As seen in Figure 11, monomer HP-POzM exhibited the maximum absorption (λ) at 550 nm, which is much higher than that of other well-known conjugated monomers, such as thiophene or 3-hexylthiophene (λ =300 nm). This absorption is redshifted due to its conjugated length in the chemical structure of HP-POzM. In the case of EHTPD, λmax was observed at 580 nm, which is higher than the absorption of HP-POzM due to the C=O linker, which has a tendency to be electron deficient. Finally, the BHBDT monomer displayed a large absorption with a λ of 600 nm, suggesting a high conjugated length in the bithiophene structure. In addition, the monomers were also measured in THF, and HP-POzM, BHBDT and EHTPD exhibited in THF of 548 nm, 530 nm and 507 nm, respectively. Based on the absorption properties of the monomer, it can be believed that monomers would be useful as acceptor/donor moieties for the synthesis of conjugated polymers used in electronic applications.

UV‒Vis absorption spectra of HP-POzM, BHBDT and EHTPD monomers in chloroform and THF.
CONCLUSION
In conclusion, monomers including acceptor/donor HP-POzM, BHBDT and EHTPD were synthesized and purified successfully in good yield. The chemical structure of the monomer has been confirmed to determine its structure. In addition, the absorption properties of HP-POzM, BHBDT and EHTPD were also investigated via UV‒Vis spectroscopy, which indicated that the monomers are potential compounds for the synthesis of novel conjugated polymers.
ACKNOWLEDGMENTS
This research was fully supported by the MURATA project under grant numbers “22VH03” and NVTX2023-20a-1.
ABBREVIATIONS
(4-hexylphenyl)(10H-phenoxazin-10-yl)methanone (HP-POzM), 4,8-bis(hexyloxy)benzo[1,2-b:4,5-b']dithiophene (BHBDT), 5-(2-ethylhexyl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione (EHTPD), Benzo[1,2-b:4,5-b' ]dithiophene (BDT), Thieno [3,4-c] pyrrole-4,6-dione (TPD), Phenoxazine (POZ), Organic field effect transistor (OFET), Organic solar cell (OSC), Organic light emitting diode (OLED), Highest-energy occupied molecular orbital (HOMO), Lowest Unoccupied Molecular Orbital (LUMO), Ultra Violet – Visible Spectrometer, Proton nuclear magnetic resonance (H NMR), Thin layer chromatography (TLC).
Conflict of interest
The authors declare that they have no competing interest.
Author contributions
Minh Duy Hoang, Bao Kim Doan and Thao Thanh Bui conceptual the project methodology wrote the original draft and supervised the investigation. Luan Thanh Nguyen, Viet Quoc Nguyen, Chau Duc Tran, Hai Le Tran and Xuan Huu Mai analysed experimental data. All authors discussed and edited the manuscript.

