Facile synthesis of carbon supported ternary nickel-cobalt-iron nanocatalyst for the hydrodechlorination of 2-chlorophenol
- University of Science, Ho Chi Minh City 70000, Viet Nam
- Vietnam National University, Ho Chi Minh City 70000, Viet Nam
Abstract
Introduction: Chlorophenols (CPs) have become prevalent in the manufacture of herbicides, fungicides, pesticides, insecticides, pharmaceuticals, and dyes, contributing significantly to water pollution. Addressing this challenge, various treatment methods such as incineration, electrochemistry, photochemistry, biotechnology, and catalytic hydrodechlorination have been explored to reduce toxic chlorophenol contaminants. Hydrodechlorination stands out as an efficient technique for the removal of chlorine atoms. The dispersion of metal nanoparticles on supported materials significantly enhances the catalytic activity by increasing the specific surface area. Among these, carbon-supported nickel-cobalt-iron nanoparticles emerge as promising catalysts for the hydrodechlorination of 2-chlorophenol.
Method: This study employs a chemical reduction method at high temperatures under an inert gas atmosphere to synthesize carbon-supported ternary nickel-cobalt-iron nanocatalysts, aiming to detoxify soil and water from pesticide residues. The physicochemical properties of these nanocatalysts were characterized using energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). Their catalytic activity was assessed through gas chromatography with a flame ionization detector (FID).
Results: The analysis revealed that metallic nanoparticles are uniformly dispersed on the carbon support, with particle sizes ranging from 10 to 60 nm and an average size of 30 nm. The hydrodechlorination (HDC) of 2-chlorophenol (2-CP) achieved satisfactory conversions under various reaction conditions.
Conclusion: The carbon-supported nickel-cobalt-iron nanoparticles demonstrated effective catalytic activity for the hydrodechlorination of 2-chlorophenol, highlighting their potential for application in the treatment of chloroorganic contaminations in the future.
Introduction
Recent developments have seen chlorophenols (CPs) extensively used in the production of herbicides, fungicides, pesticides, insecticides, pharmaceuticals, and dyes 1, 2, 3. Beyond these applications, CPs serve as preservative agents in the chemical industry for wood, paints, vegetable fibers, and leather, capitalizing on their potent antimicrobial properties4, 5, 6. Despite their utility, the adverse effects of CPs, including toxicity, carcinogenicity, teratogenicity, and mutagenicity1, 2, 7, cannot be overlooked. The U.S. Environmental Protection Agency (US EPA) has identified five CP variants as priority pollutants, with 2,4,6-trichlorophenol and 2,4-dichlorophenol listed on the Drinking Water Contaminant Candidate List (CCL)3, 7. These compounds are pervasive in groundwater, surface water, air, and soil, primarily due to improper disposal, landfill leaching, and the incineration of chlorinated waste8.
The hazardous impact of CPs on environmental and human health has spurred research into several treatment methodologies aimed at reducing the presence of these contaminants. Techniques such as incineration, electrochemistry, photochemistry, biotechnology, and catalytic hydrodechlorination (HDC) have been explored 9, 10, 11, 12. While incineration is commonly employed, it can lead to the formation of highly toxic dioxins. In contrast, methods like electrochemistry, photochemistry, and biotechnology have shown limited success. The HDC process, however, stands out for its ability to transform CPs into less harmful compounds, such as cyclohexanone and cyclohexanol, offering a non-destructive, energy-efficient solution without generating NOx/SOx emissions or dioxins/furans. HDC's effectiveness at low temperatures and its high selectivity make it a promising approach13, 14.
The HDC process typically utilizes transition-metal catalysts (e.g., Ni, Pd, Rh, Pt) in the presence of molecular hydrogen or other hydrogen sources like hydrazine, formic acid, and its salts15. Nickel, as a non-noble metal catalyst, is widely favored for various reactions but tends to suffer from issues of low selectivity and poor stability. To address these challenges, researchers have developed multimetallic catalysts combining Ni with other metals to enhance both activity and selectivity. The introduction of additional metals alters the surface properties and microstructure, introduces active sites and defects, and facilitates electron transfer through a synergistic effect, thereby improving the catalytic efficiency and stability of the materials16, 17. The choice of support material is crucial, as it provides a surface for metal nanoparticles to disperse, thereby increasing the specific surface area and influencing catalytic activity. Supports such as activated carbon, zirconium oxides, aluminum oxides, silicon oxides, magnesium oxides, titanium oxides, and zeolites have been commonly employed 7, 18. However, the high cost of palladium-based catalysts, despite their effective conversion efficiency, necessitates the development of more economically viable alternatives.
This study introduces a novel catalyst composed of ternary metallic nickel-cobalt-iron nanoparticles supported on activated carbon. These nanoparticles were meticulously characterized by physico-chemical methods before their catalytic activity was assessed through the hydrodechlorination of 2-chlorophenol (2-CP), with results presented in this report.
Materials and Methods
Materials
High-purity chemicals were utilized as received without further purification. Nickel chloride hexahydrate (NiCl·6HO, 99%), iron (II) sulfate heptahydrate (FeSO·7HO, 99%), cobalt chloride heptahydrate (CoCl·7HO, 99%), and activated carbon were procured from Xilong Chemical Co., Ltd., China. Sodium borohydride (NaBH, 98%), sodium hydroxide (NaOH, 96%), potassium hydroxide (KOH, 96%), polyvinylpyrrolidone (PVP K30), 2-chlorophenol (2-CP, 98%), and isopropyl alcohol (IPA, 99%) were obtained from various commercial sources.
Characterization
The composition of NiCoFe/C catalysts was analyzed by Energy Dispersive X-Ray (EDX) spectroscopy using a Horiba EDX 7593-H system. X-ray diffraction (XRD) patterns were obtained on a PANalytical Empyrean diffractometer using CuKα radiation (λ = 0.1541 nm, 40 kV, 100 mA), over a 2θ range of 5°–80° with a step size of 0.026°. Transmission electron microscopy (TEM) images were captured using a JEM-2100 microscope, and scanning electron microscopy (SEM) images were recorded on a Hitachi S-4800 instrument.
Catalyst Preparation
Activated carbon was pretreated following a previously established method 19. Initially, carbon powder was immersed in a 1 M HNO solution, stirring gently for 12 hours. The sample was then rinsed with deionized water until a neutral pH was achieved and dried at 80 °C for 12 hours. Subsequently, the carbon was soaked in a 1 M NaOH solution, stirred for 1 hour, rinsed, and dried under the same conditions. The final step involved calcination at 200 °C for 2 hours.
The NiCoFe/C catalyst was synthesized with modifications to the procedure described above 19. A mixture of metal salts was refluxed under a nitrogen atmosphere. Specifically, 1.2 g of PVP was dissolved in 80 mL of deionized water in a 250 mL two-neck flask, which was then heated to 80 °C. A solution containing 30 mL of NiCl·6HO, FeSO·7HO, and CoCl·7HO (each 5 mmol) in deionized water was added gradually. The mixture's temperature was increased to 100 °C, followed by the slow addition of 15 mL of a 5 M NaBH solution under vigorous stirring (700 rpm). The suspension was maintained at 100 °C for 7 hours. Subsequently, 12 g of pre-dried activated carbon was incorporated and stirred for an additional 12 hours. After washing to a neutral pH, the sample was centrifuged and vacuum-dried at 80 °C for 24 hours to yield the catalyst as a black powder.
Catalyst Evaluation
The catalytic activity of the carbon-supported ternary NiCoFe nanoparticles was assessed in the liquid-phase hydrodechlorination of 2-CP under alkaline conditions. A reaction mixture containing 4 mmol of naphthalene (internal standard), 4 mmol of 2-CP in 50 mL of IPA, and 4 mmol of KOH was prepared in a 250 mL two-neck flask. After adding a predetermined amount of the catalyst, hydrogen gas was introduced, and the reaction was conducted at various temperatures and durations. The reaction mixture was centrifuged, and substrate conversion was analyzed using an Agilent GC-FID equipped with an HP-5 capillary column (30 m × 0.320 mm).

Hydrodechlorination of 2-chlorophenol
Results
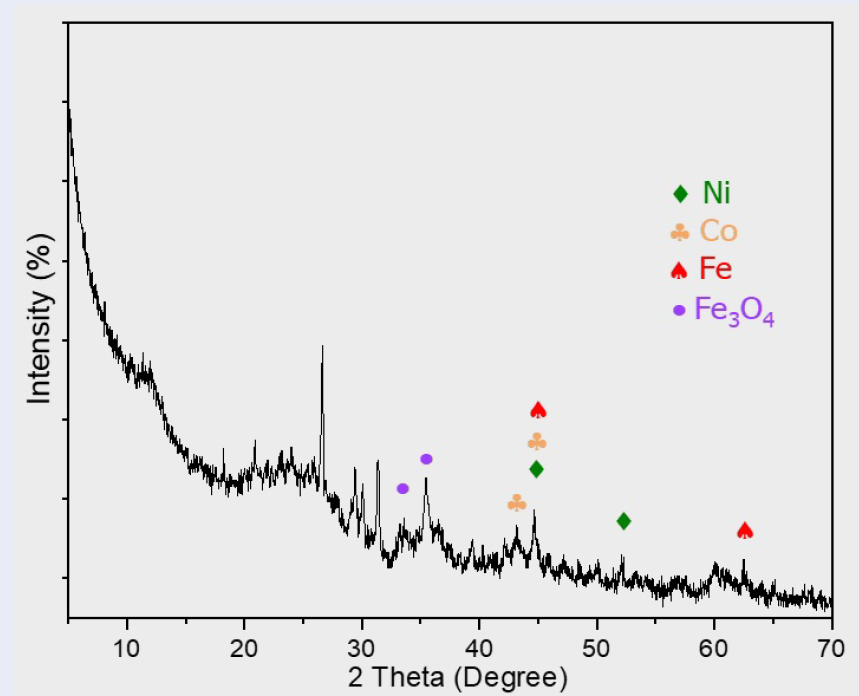
The X-ray diffraction (XRD) analysis of the NiCoFe/C catalyst, as depicted in Figure 2, revealed characteristic diffraction peaks indicating the presence of nickel, cobalt, and iron nanoparticles. Specifically, nickel exhibited peaks at 2θ values of 44.64° and 52.07°. Cobalt was identified by peaks at 2θ values of 43.19° and 44.64°, while iron's presence was confirmed through peaks at 44.64° and 62.47°. Additionally, iron oxide signals were observed at 2θ values of 33.58° and 35.44°, likely resulting from the oxidation of iron particles to iron oxides (Fe and Fe) due to air exposure. Impurities, potentially silicon, were indicated by peaks at 2θ values of 26.65°, 29.38°, 30.09°, and 31.40°.

XRD patterns of NiCoFe/C catalyst.
Energy-dispersive X-ray (EDX) spectroscopy analysis (Figure 3) confirmed the chemical composition of the synthesized catalysts, validating the presence of Ni, Co, and Fe with mass percentages closely aligning with theoretical values. This indicates a successful impregnation process. The analysis also suggested a near-complete removal of metallic impurities from the activated carbon, with a minor silicon trace detected (0.82 wt%).

EDX spectra of NiCoFe/C catalyst.
Scanning electron microscopy (SEM) images (Figure 4a) highlighted the catalyst's rough and porous surface morphology, advantageous for nanoparticle loading and indicative of a substantial specific surface area. This morphology is expected to enhance hydrodechlorination (HDC) conversion efficiency relative to untreated carbon. Transmission electron microscopy (TEM) (Figure 4b) revealed spherical nanoparticles ranging from 10 nm to 60 nm in diameter, averaging 30 nm. These nanoparticles showed uniform spherical shapes and excellent dispersion across the carbon support without agglomeration, particularly of the magnetic Fe nanoparticles, indicating successful active site dispersion within the carrier matrix.

Morphological surface of NiCoFe/C catalyst: a) SEM image captured at 10 kV, 8.1 mm WD, and 5 μm scale; b) TEM images captured at 200 nm scale.
Discussion
The X-ray diffraction (XRD) analysis (Figure 2) revealed residual unreduced iron oxide within the catalyst sample. Nonetheless, this presence is unlikely to detract from catalytic performance, as the primary function of iron and cobalt is to enhance the dispersion of nickel's active sites and increase the adsorption of hydrogen and the substrate. This facilitates a faster and more efficient reaction 20. This assertion is supported by the scanning electron microscopy (SEM) images (Figure 4a), which depict an increased surface roughness and development of porous, interpenetrating networks on the catalyst. Additionally, transmission electron microscopy (TEM) images (Figure 4b) indicate that the ternary nickel-cobalt-iron nanoparticles are larger on average (30 nm) compared to single-metal nickel nanoparticles (15 nm).
The hydrodechlorination (HDC) activity of 2-chlorophenol (2-CP) was assessed across varying nickel contents (2, 5, and 10 wt%), reaction times, and temperatures. The findings show that an increase in catalyst concentration leads to higher conversion rates, attributable to the augmented number of Ni active sites and consequently, a more efficient H bond-breaking process, enhancing HDC reaction efficiency.

Influence factors to the conversion of HDC of 2-CP, reaction condition: y% mol catalyst, in the solution of KOH 0.08 M in iso-propanol: a) the conversion vs time, reaction temperature: 30 C; and b) temperature, reaction time: 6h.
Figure 5 illustrates the factors influencing the conversion efficiency in HDC of 2-CP, under the reaction conditions of y% mol catalyst in a 0.08 M KOH solution in isopropanol. An increase in reaction time from 1 to 3 hours resulted in improved conversions across all catalytic entries (Figure 5a). Specifically, conversions rose from 13.5% to 15.2% for the Cat2% sample, from 16.6% to 20.5% for the 5% sample, and from 18.7% to 25.0% for the Cat10% sample, likely due to enhanced adsorption on the catalyst surface, thereby boosting the HDC activity.
However, extending the reaction to 6 hours led to a decrease in conversion for all three samples, potentially due to catalyst poisoning by hydrochloric acid produced during the reaction or the blockage of active sites by large organic molecules. Additionally, long-term reactions might lead to partial washing or inactivation of the metal nanoparticles. Interestingly, while the Cat2% and Cat10% samples showed increased conversion after 24 hours, the Cat5% sample experienced a slight decrease. This could suggest that catalyst leaching might occur over extended reaction times, affecting activity variably across different catalyst concentrations. The presence of potassium hydroxide may neutralize produced HCl, mitigating catalyst poisoning and favoring the reaction's forward progression.
Furthermore, Figure 5b explores the temperature's impact on 2-CP conversion in HDC. Increasing the reaction temperature to 60 °C resulted in reduced conversion rates. For instance, using a 15% catalyst concentration, the activity significantly dropped from 31.8% at 30°C to 20.5% at 60 °C. This decrease can be attributed to high-temperature-induced desorption, as described by the Arrhenius equation 21, leading to the release of hydrogen gas and 2-CP substrates from the nickel active sites.
Typical hydrodechlorination of 2-chlorophenol reported in the literature.
|
No |
Catalyst |
Method |
Weight (mol%) |
Time (min) |
Temperature (°C) |
Conversion (%) |
[Ref] |
|
1 |
Pd/Fe |
HPLC |
1 |
300 |
30 |
88 |
|
|
2 |
5%Pd/C |
GC |
1 |
70 |
30 |
98 |
|
|
3 |
5%PdZn/CNT |
HPLC |
1 |
75 |
100 |
100 |
|
|
4 |
15%NiCoFe/C |
GC |
2 |
360 |
30 |
35 |
This work |
Conclusions
This study successfully synthesized ternary metallic nanoparticles composed of nickel, cobalt, and iron, supported on activated carbon, with their physicochemical properties thoroughly characterized. The results revealed that these metallic nanoparticles were well-dispersed across the surface of the carbon supports, enhancing the catalyst's overall effectiveness. Notably, the catalysts demonstrated significant catalytic activity in the hydrodechlorination of 2-chlorophenol, even with relatively low nickel content. This finding is particularly compelling as it suggests that these nickel-based catalysts could potentially serve as cost-effective alternatives to the more expensive and noble palladium catalysts traditionally used in this application.
Moreover, the promising outcomes of this research not only contribute to advancing the field of catalysis but also hold significant implications for the water pollution treatment industry. By offering a more affordable catalyst option, this work paves the way for broader development and application in industrial water treatment processes, ultimately contributing to the mitigation of water pollution challenges.
ABBREVIATIONS
Cat2%: 2% of metal content in the sample
COCs: Chlorinated organic compounds
2-CP: 2-chlorophenol
DI: Deionized
FID: flame ionization detector
HDC: Hydrodechlorination
AUTHOR’S CONTRIBUTIONS
Thuan Khiet Trinh Nguyen, Nguyen Minh Tam Phan, Thi My Duyen Truong, Thao Vy Duong, and Thi Duyen Diep did the experiments on catalytic synthesis and chacracterized the samples. Thi Yen Nhi Nguyen and Thanh Thien Co carried out the catalytic activity of HDC reaction, analysed the results, and wrote the manuscript. All authors read and approved the final manuscript.
FUNDING
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number B2023-18-03.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest regarding the publication of this paper.

