Synthesis of Co3O4 electrodes by electrochemical deposition for water splitting reaction
- University of Science, VNU-HCM, 227 Nguyen Van Cu Street, District 5, Ho Chi Minh City 700000, Viet Nam
- Viet Nam National University Ho Chi Minh City, Thu Duc City, Ho Chi Minh City, Viet Nam
- Advanced Materials and Applications Research Group (AMA), HUTECH University, 475A Dien Bien Phu Street, Binh Thanh District, Ho Chi Minh City 700000, Viet Nam
Abstract
Introduction: The use of Ni foam substrates for the growth of catalysts is a common practice in electrochemical water splitting reactions, although their stability in some electrolytes can be problematic, hindering the scalability of synthesis. This study aims to explore alternative substrates for catalyst growth, focusing on cobalt oxide (Co3O4) due to its potential in enhancing electrochemical water splitting efficiency.
Methods: Cobalt oxide (Co3O4) was synthesized on various conductive substrates including fluorine-doped tin oxide (FTO), indium-doped tin oxide (ITO), and carbon cloth (CC), employing electrochemical deposition techniques. The morphological and crystalline properties of the Co3O4 coatings on these substrates were characterized and analyzed to understand their influence on the catalyst's performance in water splitting reactions.
Results: The electrochemical deposition resulted in a more condensed coverage of Co3O4 on the CC substrate, attributed to the crystal's oriented aggregation. The crystallization and lattice development of Co3O4 varied significantly across different substrates, exhibiting high crystallization on FTO and ITO substrates but poorer crystallization on the CC substrate. Notably, the Co3O4/CC electrode demonstrated superior performance in hydrogen evolution reaction, achieving the lowest overpotential of -382 mV at a current density of 10 mA cm-2.
Conclusion: The findings suggest that carbon cloth (CC) presents a promising alternative to Ni foam substrates for the growth of Co3O4 catalysts in electrochemical water splitting applications. The enhanced performance of Co3O4/CC electrodes, particularly in terms of overpotential and crystallization behavior, highlights the potential of using CC substrates to improve the efficiency and scalability of water splitting reactions for sustainable hydrogen production.
Introduction
Cobalt oxide (CoO) has emerged as a prominent catalyst in electrochemical water splitting (EWS) applications, demonstrating significant efficacy in both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) due to its impressive charge transfer capabilities and large surface area1, 2, 3, 4, 5. Recently, Liu . synthesized CoO quantum dots and combined them with TiO materials to improve the efficiency of charge transfer between the two materials to increase the water-splitting activity of the material6. Similarly, Yuan et al. have developed a composite material combining CoO with nitrogen-doped carbon, supported on Ni foam, to facilitate comprehensive water-splitting for both HER and OER reactions. Despite CoO's efficiency, the use of Ni foam substrates has been noted to pose stability issues in acidic environments, suggesting a preference for basic conditions for optimal water splitting reactions7.
Recently, La developed CoO/CC for overall water splitting and demonstrated that this material has an efficient catalytic performance8, 9. Furthermore, CoO/CC also expressed a high ESW performance at a NaSO electrolyte that does not react or interfere with most of the target electrode or electrochemical reactions, respectively10. Moreover, NaSO solution is a stable electrolyte over electrodes based on SnO-glass 11.
Various methods, including hydrothermal synthesis, sol-gel, electrochemical deposition, and sputtering, have been explored for synthesizing CoO on substrates12, 13, 14, 15, 16, 17. Among these, electrochemical deposition stands out as a straightforward, eco-friendly approach that minimizes chemical use and production time. This method is especially beneficial for fabricating electrodes for EWS reactions, which necessitate the integration of catalysts with conductive substrates to ensure efficient electron transfer. The selection of an appropriate conductive electrode—be it graphite, carbon-based materials like carbon nanotubes (CNTs) or carbon cloth (CC), metal foams or meshes, conductive polymers, or transparent conductive oxides such as FTO and ITO—is crucial for optimizing EWS efficiency and paving the way for future practical applications18, 19.
This study aims to synthesize CoO on various conductive substrates (FTO, ITO, and CC) using electrochemical deposition under low potential and temperature conditions. Characterization of the CoO coatings was conducted via X-ray diffraction (XRD) patterns and SEM observations. Furthermore, a NaSO solution was used as an electrolyte for the EWS evaluation over catalytic electrodes.
Methods
Materials
The following chemicals and materials were utilized: Cobalt (II) nitrate hexahydrate (Co(NO)·6HO, GHTECH, 98.6%), ethylene glycol (EG, CHO, Xilong, > 98%), potassium hydroxide (KOH, Merck, 99%), sulfuric acid (HSO, Xilong, > 98%), commercially available carbon cloth (Viet Nam), deionized (DI) water (18 MΩ.cm), and ethanol (CHOH, Thermo Fisher Scientific, 99%).
Electrochemical Deposition Synthesis of CoO
Initially, 2.91 g of Co(NO)·6HO was dissolved in 100 mL of DI water to prepare a 0.1 M Co(NO) solution. Substrates including FTO, ITO, and CC were cleansed using DI water and ethanol through ultrasonication sequentially, followed by oven-drying. Electrodeposition was performed in a standard three-electrode system comprising the conductive electrode (FTO, ITO, or CC), a Pt wire, and an Ag/AgCl electrode (in saturated KCl solution) as the working electrode, counter electrode, and reference electrode, respectively. The procedure was conducted for 5 minutes at a constant voltage of -1.0 V, as previously described8. The process resulted in the formation of blue precipitate, presumed to be Co(OH), on the working electrodes. The Co(OH)/CC obtained was oven-dried and subsequently annealed in air at 400°C for 2 hours to yield CoO-decorated electrodes, following optimized parameters from our earlier study 8.
Characterizations
XRD was employed to ascertain the crystalline phases of the materials, utilizing a Bruker D8 instrument with a Cu Kα radiation source (λ = 1.5406 Å), an electron accelerating voltage of 45 kV, current of 45 mA, and a scanning rate of 0.02°. Surface morphology was examined using a JSM-IT500 scanning electron microscope (JEOL), with samples being Au-coated prior to insertion into the measurement chamber. An electron accelerating potential of 20 kV was applied for all SEM imaging. Elemental distribution and quantification were conducted via EDX mapping with the Oxford Instruments.
Linear Sweep Voltammetry Experiments
Linear sweep voltammetry (LSV), a potentiometric method measuring current while linearly scanning potential over time, was utilized to identify oxidation or reduction peaks indicative of HER and OER activities. CoO-decorated substrates (1 cm total area) were evaluated in a standard three-electrode system using a Biologic SP-200 potentiostat. LSV was conducted at a scan rate of 10 mV s in 1.0 M NaSO solution, with Ag/AgCl (saturated KCl) as the reference electrode and Pt wire as the counter electrode. HER polarization curves were recorded from -0.5 V to -1.5 V (vs. Ag/AgCl), and OER polarization curves from -1.0 V to 1.0 V (vs. Ag/AgCl) 20.
Results
X-ray Diffraction Analysis
The XRD patterns of CoO decorated on various substrates are depicted in Figure 1. The patterns reveal peaks corresponding to the underlying substrates of CC, FTO, and ITO. Additionally, distinct peaks at 2θ values of 19°, 31.2°, 36.8°, 44.8°, 59.3°, and 65.2° are observed, which correlate with the (111), (220), (311), (400), (511), and (440) lattice planes of CoO, respectively [JCPDS #80-1532]. Notably, the (440) and (311) planes of CoO are prominently featured when deposited onto FTO and ITO substrates, respectively. Conversely, the crystalline intensity of CoO on the CC substrate appears to be weaker.

XRD patterns of Co3O4 decorated on different substrates.
Scanning Electron Microscopy Observations
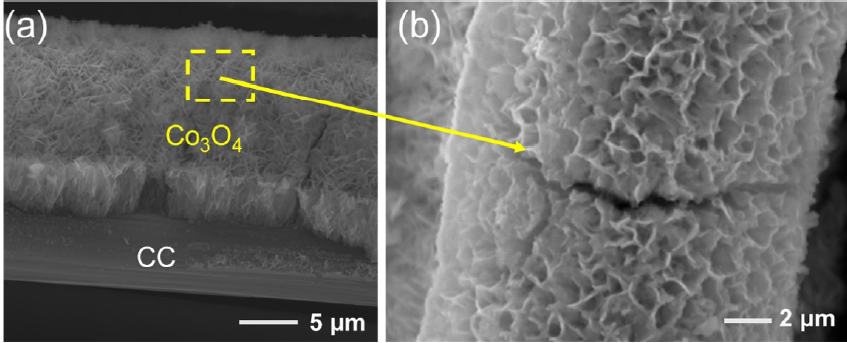
SEM analysis was conducted to examine the morphology of CoO on the substrates, as illustrated in Figure 2, Figure 3, Figure 4. Figure 2 presents CoO/ITO with a porous structure distinctly different from the smooth structure of the underlying ITO. Figure 3 highlights the morphology of CoO/FTO, indicating slight variations with non-uniform CoO particle sizes. In contrast, Figure 4 shows CoO/CC densely covered with a uniform layer of CoO, approximately 4-5 µm thick, exhibiting a consistent porous structure across the CC substrate.

SEM images of Co3O4/ITO with different scales: (a) 10 µm, (b) 5 µm.

SEM images of Co3O4/FTO with different scales: (a) 10 µm, (b) 5 µm.

SEM images of Co3O4/CC with different scales: (a) 5 µm, (b) 5 µm.
Linear Sweep Voltammetry Analysis
LSV measurements were utilized to evaluate the EWS activity of CoO on various substrates, as depicted in Figure 5. The HER performance, showcased in Figure 5(a), reveals CoO/CC as the most active electrode, achieving the lowest onset potential of -382 mV at a current density of 10 mA cm. Comparatively, CoO/FTO and CoO/ITO require significantly higher overpotentials of -975 mV and -1610 mV, respectively, to reach -10 mA cm, underscoring the superior HER efficiency of CoO/CC.
OER activities, shown in Figure 5(b), indicate that none of the electrodes—FTO, ITO, or CC—exhibit effective EWS performance for OER, as measured in 1.0 M NaSO at a scan rate of 10 mV s. This suggests that while CoO/CC demonstrates promising HER capabilities, improvements are needed to enhance OER performance across all evaluated substrates.

LSV plots of Co3O4 decorated on different substrates for HER (a) and OER (b) processes.
Discussion
XRD patterns depicted in Figure 1 reveal distinctive crystal structures among the conductive substrates used. Notably, both FTO and ITO exhibit high crystallinity, with predominant orientations at the (110) and (222) planes, respectively. This variance in diffraction peaks can be attributed to the unique crystalline characteristics of each substrate, which in turn influence the nucleation and growth of CoO during the electrochemical deposition process. Specifically, the pronounced crystalline growth of CoO along the (440) and (311) planes on FTO and ITO substrates, respectively, contrasts with the weaker crystal intensity observed on the CC substrate. However, the presence of both (440) and (311) planes of CoO on the CC substrate suggests a nuanced texture development that directly impacts CoO's morphology21.
The observed disparities in CoO crystal growth across different substrates might be elucidated by oriented aggregation phenomena, where crystals tend to cluster around emerging seed sites. This process results in a non-uniform size distribution of CoO, as evidenced by the SEM images in Figure 4. These images further demonstrate that the CC substrate facilitates a more uniform distribution of CoO compared to FTO and ITO substrates, enhancing the HER performance of CoO.
Moreover, the electrochemical measurements highlighted in Figure 5 and
The comparative analysis of overpotentials for HER and OER across CoO-decorated substrates reveals significant variations, possibly due to differences in electrolyte environments. While prior studies predominantly employed 1.0 M KOH, this research utilized 1.0 M NaSO, showcasing the CoO/CC electrode's superior activity and lower overpotential in comparison to other electrodes. This suggests that the choice of electrolyte can markedly influence the EWS performance of CoO electrode systems, with the CoO/CC configuration exhibiting enhanced activity at reduced overpotentials.
Overpotential at 10 mA cm-2 of Co3O4 decorated on different substrates in 1.0 M Na2SO4 solution
|
Material |
Substrate |
Synthesis methods |
HER (mV) |
OER (mV) |
Electrolyte |
Ref. |
|
Co3O4 |
Pt/ SiO2/Si |
pulsed laser deposition |
263 |
402 |
1.0 M KOH |
|
|
Co3O4 |
N doped carbon |
heat treatment |
237 |
401 |
1.0 M KOH |
|
|
Co3O4 |
Nickel foam |
hydrothermal |
316 |
520 |
1.0 M KOH |
|
|
Co3O4 clusters |
Ag |
laser ablation |
51 |
206 |
1.0 M KOH |
|
|
Co3O4−δ quantum dots |
Carbon fiber paper |
lithiation/delithiation |
- |
270 |
1.0 M KOH |
|
|
Co3O4 |
polypyrrole/MWCNT |
mechanical mixing and heat treatment |
490 |
340 |
1.0 M KOH |
|
|
Co3O4 |
Ti3C2Tx MXene@Nickel foam |
Electrodeposition |
350 |
520 |
1.0 M KOH |
|
|
Co3O4 |
CC |
Electrodeposition |
382 |
1130 |
1.0 M Na2SO4 |
This study |
|
Co3O4 |
ITO |
Electrodeposition |
1610 |
- |
1.0 M Na2SO4 |
This study |
|
Co3O4 |
FTO |
Electrodeposition |
975 |
1340 |
1.0 M Na2SO4 |
This study |
Conclusion
In summary, we has successfully synthesized CoO on various substrates, including FTO, ITO, and CC, through an electrochemical deposition, as confirmed by XRD and SEM analyses. The findings reveal that CoO exhibits distinct (440) and (311) planes when deposited on the CC substrate, which significantly influences its morphology. Notably, the CoO/CC electrode demonstrates superior EWS performance in both HER and OER. Specifically, the CoO/CC electrode achieved the lowest observed overpotentials of -382 mV for HER and 1130 mV for OER at a consistent current density of 10 mA cm, utilizing a 1.0 M NaSO electrolyte. These results underscore the potential of CoO/CC as a highly effective catalyst for EWS applications, highlighting its promising capacity for energy conversion processes. The study paves the way for further exploration into the optimization of CoO-based electrodes for sustainable hydrogen and oxygen production.
ACKNOWLDEDMENTS
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under Grant B2022-18-03.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.

