Investigate the influence of a CagA-negative Helicobacter pylori strain, GD13, on nonhomologous end joining-mediated repair of proximal DNA double-strand breaks in GCV6 cells
- VNUHCM-University of Science, Department of Genetics, Faculty of Biology and Biotechnology, Ho Chi Minh City, Vietnam
- Department of Genetics, Faculty of Biology and Biotechnology, University of Science, Ho Chi Minh City, Vietnam
- Cancer Research Laboratory, University of Science, Ho Chi Minh City, Vietnam
- Viet Nam National University Ho Chi Minh City, Viet Nam
Abstract
Mammalian cells infected with Helicobacter pylori show an accumulation of DNA double-strand breaks (DSBs). Previous studies have shown that the virulence factor CagA of H. pylori induces genomic instability in host cells. Most DSBs in infected cells are repaired by nonhomologous end-joining (NHEJ) pathways. Indeed, impairment of NHEJ-mediated DSB repair may lead to genetic alterations and contribute to the development of gastric cancer. Nonetheless, the impacts of H. pylori and its virulence factor, CagA, on the efficacy of NHEJ are poorly defined. This study aimed to reveal the effect of GD13, a CagA-H. pylori strain isolated in Vietnam, on the NHEJ-mediated repair of DSBs in host cells. For this purpose, we utilized GCV6 cells with an NHEJ-reporter substrate, which has been proven to be an appropriate model for evaluating the repair efficiency of NHEJ in H. pylori-infected cells. The level of interleukin-8 released into the cell culture supernatant was measured by ELISA. Our findings suggest that the reference CagA+ 26695 strain impaired the NHEJ-mediated repair of double-strand breaks (DSBs), whereas the GD13 strain has not exhibited any discernible effect thus far. In addition, H. pylori strain GD13 triggered a lower level of interleukin-8 secretion in GCV6 cells than did the CagA-positive strain 26695.
INTRODUCTION
infection contributes to 75-98% of all gastric cancer cases 1. Gastric cancer is the fifth most common cancer and the fourth leading cause of cancer-related mortality 2. Preventing the onset of cancer largely depends on ensuring genome stability via high-fidelity DNA damage repair pathways. DNA double-strand breaks (DSBs) are the most lethal form of DNA damage. Two major DSB repair pathways in human cells are homologous recombination (HR) and nonhomologous end joining (NHEJ) 3. Because DSBs are mainly induced in the G1 phase in -infected cells, the NHEJ pathway, which is active throughout the cell cycle, is the predominant DSB repair pathway in these cells4, 5, 6. During NHEJ, two DNA ends are directly religated without relying on a DNA template. There are two NHEJ subpathways: classical NHEJ (cNHEJ) and alternative NHEJ (aNHEJ). While cNHEJ preserves genome integrity, aNHEJ generates more erroneous repairs and fosters the loss and translocation of chromosomes 3. Microhomology is a feature of aNHEJ and is identified at rearrangement breakpoints in various malignancies7.
The pathogenicity of is associated with alterations in host signaling pathways. infection induces the release of interleukins, TNF-alpha, and interferon, which mediate the innate immune response in host cells 8. In vivo and in vitro studies have demonstrated that cells infected with have elevated IL-8 secretion 9. In addition, NF-κB plays a dominant role in -induced IL-8 production in gastric epithelial cells 10. The most well-known virulence factor of is Cytotoxin-associated gene A (CagA). strains can be divided into CagA-positive (CagA) or negative (CagA) strains. The bacteria deliver CagA into gastric epithelial cells via the bacterial type IV secretion system (T4SS). CagA promotes the malignant transformation of cells, including elevated cell motility, sustained proliferation, resistance to cell death, and genomic instability 11. Many studies have demonstrated the impact of CagA on HR-mediated DSB repair. CagA reduces the expression of factors involved in HR pathways, destabilizes replication forks, and causes replication-induced DSBs to accumulate in host cells12. By upregulating a long noncoding RNA, SNHG17, CagA inhibits the expression of RAD51 13. Furthermore, CagA hinders PAR1b-mediated BRCA1 phosphorylation and subsequently subverts the nuclear translocation of BRCA1. Consequently, HR activity is suppressed, and DSBs accumulate in -infected cells14. also enhances the accumulation of DSBs in gastric tissue cells through CagA-independent mechanisms. In this regard, two metabolic precursors of lipopolysaccharide biosynthesis in are HBP (D-glycero-beta-D-manno-heptose 1,7-bisphosphate) and β-ADP-heptose (ADP-beta-D-manno-heptose), which activate the ALPK1/TIFA/NF-kB signaling pathway. Activated NF-kB enhances transcriptional activity during the S phase, when replication is ongoing, leading to the formation of DNA/RNA hybrids (R-loops). This structure further results in replication fork stalling and single-strand DNA gaps, which are the source of DSBs 15. Currently, the effects of on NHEJ-mediated DSB repair in both CagA-dependent and CagA-independent manners remain poorly understood.
We recently developed a procedure using the GCV6 cell model carrying an NHEJ-reporter construct to quantitatively assess the efficiency of NHEJ repair when cells are infected with . In addition, we found that 5 Cag strains have distinct effects on the DSB repair efficiency of cells and speculated that infection can subvert the NHEJ repair system via a CagA-independent mechanism16. In this study, we utilized this cell model to examine the influence of a Vietnamese CagA strain, GD13, and 26695, a standard reference CagA strain.
MATERIALS AND METHODS
strains and typing of cagA/vacA genes by multiplex PCR
The strain 26695 (ACTC 26695/NCTC 26695) was used as a standard reference. The GD13 strain was derived from a urea test-positive gastric biopsy sample of a gastritis patient at Gia Dinh Hospital (Vietnam) 17. The isolation and culture conditions were as previously described 16. DNA was extracted from each strain and amplified by multiplex PCR to characterize the cagA/vacA genotype18. The strain Tx30a (ATCC 51932), with the genotype , s2/m2, was used as a positive control for the empty site of .
Measuring the efficiency of NHEJ
The following cell culture conditions were used: transfection, culture and infection, and NHEJ 16. Briefly, GCV6 cells were first transfected with the I-SceI expression vector (pBASce) for 24 h. Next, the cells were infected with GD13 or 26695 strains at a multiplicity of infection (MOI) of 10 or 50. Twenty-four hours and 48 hours later, the cells were harvested in 1× PBS and fixed with 4% paraformaldehyde for 20 min at 4°C. The percentage of GFP-positive cells was analyzed in a population of at least 3000 viable cells and corrected with pBASce-nontransfected samples using a BD Accuri C6 plus flow cytometer (BD).
Quantification of interleukin-8 secretion
After 24 h of infection with the GD13 and 26695 strains at an MOI of 50, we separated 1 mL of cell culture medium and centrifuged it to collect the supernatant. The IL-8 concentration in the cell culture medium was measured with an enzyme-linked immunosorbent assay (ELISA) using a Human IL-8 Uncoated ELISA (Invitrogen, 88-8086) according to the manufacturer’s instructions. For technical replication, each sample was quantified in two separate wells.
Statistical analysis
All biological results are expressed as the mean ± standard deviation of three independent experiments. Normally distributed data were analyzed with the Shapiro‒Wilk test and Student’s t test using GraphPad Prism 8.2.0. The statistical significance of differences was indicated by a p value < 0.05.
RESULTS
Detection of cagA gene and typing of vacA gene
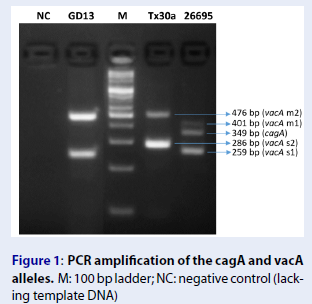
We performed multiplex PCR to identify the genotype of the GD13 strain. The lengths of the target fragments on , s1/s2, and m1/m2 were 349 bp, 259 bp/296 bp, and 401 bp/476 bp, respectively. The TX30A strain (/ s2m2) and 26695 strain (/ s1m1) were used as positive controls19, 20. The electrophoresis of PCR products on a 5% agarose gel showed that the GD13 strain harbors s1m2 and does not harbor CagA (Figure 1).

PCR amplification of the cagA and vacA alleles. M: 100 bp ladder; NC: negative control (lacking template DNA)
Quantification of IL-8 secretion
To determine whether infection results in the induction of IL-8 in GCV6 cells, we measured the amount of IL-8 released into the cell culture supernatant 24 h after infection with 26695 and GD13 at an MOI of 50. As presented in Figure 2, both strains can trigger IL-8 secretion. The amount of IL-8 was 108-fold greater in cells infected with the 26695 strain (9865 ± 1476 pg/mL) and approximately sixfold greater in GD13 strain-infected cells (509 ± 68.43 pg/mL) than in noninfected cells (88.76 ± 31.47 pg/mL). Furthermore, the GD13 strain induced significantly less IL-8 than did the 26695 strain.

The concentration of IL-8 in the cell culture supernatant was detected by ELISA. Control: noninfected cells. The data are presented as the means ± SDs. n = 3. ***p <0.001,2-tailed Student’s t test.
Measurement of NHEJ efficiency in DSB repair
Previously, we designed an experimental protocol to assess the effect of on NHEJ using the GCV6 cell model (16). Each cell harbors a single copy of an NHEJ-reporter substrate stably inserted into its genome (Figure 3A). The reporter contains two recognition sites of endonuclease I-SceI separated by 34 bp. Due to an upstream and out-of-frame start site, “Koz-ATG”, green fluorescent protein (GFP) cannot be synthesized in cells. The release of “Koz-ATG” following I-SceI expression and NHEJ repair of I-SceI-induced DSBs generates GFP-positive (GFP) cell. Measuring the percentage of GFP cells in the population by flow cytometry allowed us to determine the rejoining efficiency of NHEJ-mediated repair DSBs (Figure 3B).

Model for assessing the efficiency of NHEJ-mediated repair of DSBs. (A) The schematic diagram represents the NHEJ substrate in the GCV6 cell line. (B) NHEJ efficiencywas assessedby the percentage of GFP. The cell population was gated based on diameter (FSC) and internal complexity/granularity (SSC). GFP was suppressed without I-SceI expression in contrast, when I-SceI was transfected into the cells, the sequence of Koz ATG was cleaved, the DNA ends were religated, GFP was expressed, and a GFP-positive cell ratio was detected.
Here, we infected the cells with 26695 and GD13 at MOIs of 10 and 50 for 24 h and 48 h. The flow cytometry data in Figure 4 show that at 24 h postinfection, the percentage of GFP-positive cells at both an MOI of 10 (0.78 ± 0.08) and an MOI of 50 (0.82 ± 0.04) was lower in the 26695-infected group than in the noninfected group. The efficiency of NHEJ decreased even further after 48 h of infection (MOI 10: 0.71 ± 0.06; MOI 50: 0.70 ± 0.04).
Unexpectedly, in this study, we observed that there was no significant difference in the percentage of GFP-positive cells between cell populations infected with GD13 and the noninfected group (M10 at 24 h: 1.05 ± 0.09; M10 at 48 h: 1.12 ± 0.27; M50 at 24 h: 1.03 ± 0.11; M50 at 48 h: 1.02 ± 0.41) (Figure 4). Thus far, the results indicated that CagAGD13 does not affect the repair of DSBs by NHEJ.

The efficiency of NHEJ-mediated DSB repair in GCV6 cells after 24 h and 48 h of
DISCUSSION
In this study, we observed the impact of GD13, a CagA strain, on the NHEJ efficiency of host cells compared to that of the standard CagA strain 26695. IL-8 is a potent chemoattractant that stimulates the infiltration of neutrophils into the gastric mucosa9. We found that IL-8 upregulation can be observed in both GCV6 cell populations infected with two strains. Notably, the 26695 strain promoted significantly more IL-8 than did the GD13 strain. Our data are consistent with those of previous studies showing that CagA is a determining factor in the induction of IL-8 release and that CagA induces IL-8 in fibroblasts via a pathway similar to that in AGS cells21, 22. Our data also showed that NHEJ-mediated repair of DSBs decreased in cells infected with the 26695 strain but remained at a similar level in cells infected with the GD13 strain. This result is in line with the findings of other groups and our studies, which have indicated that strains with impair NHEJ by stimulating aNHEJ and suppressing cNHEJ activity13, 16, 23, 24. Our results suggest that the effect of on stimulating inflammatory responses and NHEJ efficiency in host cells might be associated with infection. As we have demonstrated the diverse impact of CagA strains on both IL-8 induction and DSB repair efficiency, the effect of additional CagA strains should be investigated. Moreover, the GFP ratio does not fully reflect the rate of successful repair by the NHEJ mechanism. Upon I-SceI expression, a DSB can be induced at either or both I-SceI sites. If “Koz-ATG” does not pop out or the GFP reading frame is not corrected, GFP cannot be expressed. Moreover, if the GFP sequence and/or the regulatory promoter of this gene are shortened during the repair process, the expression of GFP is also silent, even though the break has been repaired. Therefore, the DNA sequence at repair junctions should be analyzed in further studies.
CONCLUSIONS
Our data indicated that CagAGD13 stimulated the inflammatory response by inducing interleukin-8 secretion in GCV6 cells to a lesser extent than the CagA 26695 strain. Using GCV6 cells with an NHEJ-reporter substrate, we found that strain 26695 diminished the NHEJ-mediated repair of DSBs, while the GD13 strain has not yet shown any effect. Examining the impact of more CagA strains, comparing CagA-deficient strains with CagA-proficient strains in an isogenic background, and characterizing the repair junctions at the NHEJ reporter should be the focus of future studies to enhance our understanding of the mechanism of infection carcinogenesis through the induction of genetic instability in host cells.
LIST OF ABBREVIATIONS
:
DSB: DNA double-strand break
NHEJ: Nonhomologous end joining
aNHEJ: Alternative nonhomologous end joining
cNHEJ: Classical nonhomologous end joining
HR: Homologous recombination
CagA: Cytotoxin-associated gene A
GFP: Green fluorescent protein
IL-8: Interleukin 8
MOI: Multiplicity of infection
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
This research is funded by the University of Science, VNU-HCM under grant number SH-CNSH 2022-11.
We thank Dr. Benard Lopez and Dr. Guirouilh-Barbat (Center National de la Recherche Scientifique UMR 8200, Villejuif, France) for providing us with the GCV6 cell line.

