Biosynthesis of Staphylococcus aureus OS-silver nanoparticles and their antimicrobial and protective effects on coated paper money
- Department of Microbiology, The Federal University of Technology, PMB 704, Akure, Nigeria
- Department of Microbiology, The Federal University of Technology, PMB 704, Akure, Nigeria,Department of Agricultural Science, Adeyemi Federal University of Education, Ondo, Nigeria
- Department of Food Science and Technology, Olusegun Agagu University of Science and Technology, PMB 353, Okitipupa, Nigeria
Abstract
Introduction: Currency notes harbor and transmit infectious microorganisms if handled without proper care. Hence, in this study, silver nanoparticles biosynthesized from autochthonous S. aureus OS (S-AgNPs) isolated from Nigeria currency notes (Naira) were used to assess the antimicrobial activity of S-AgNPs against the pathogenic money microbiome and to determine their protective effect on coated currency notes.
Methods: Naira notes (15) each, were randomly collected from poultry product sellers, food vendors, fish, sellers, and shopkeepers in Ondo City, Nigeria. A molecular tool was used for the identification of bacterial isolates using 16S ribosomal RNA gene sequences. Staphylococcus aureus-silver nanoparticles (S-AgNps) were characterized by UV‒visible spectroscopy. The antibacterial and antifungal activities of the biosynthesized S-AgNPs against isolated microorganisms were determined using the agar well diffusion method.
Results: A total of one hundred and twenty (120) S-AgNP-coated and non-coated papers were improvised as currency notes and randomly distributed among preinformed business owners. The highest bacterial count of 7.90 cfu/mL was recovered from Naira notes collected from food vendors. The highest fungal count of 4.70 cfu/mL on the Naira note was collected from poultry product sellers. S. aureus had the highest frequency of 29.60%. At 5.0 µg/mL, the S-AgNPs had the greatest inhibitory effects (17.30 mm) on Streptococcus pyogenes and Rhizopus stolonifer. The provided currency notes coated with the biosynthesized S-AgNPs showed no growth of microorganisms. Biosynthesized S-AgNPs showed pronounced antimicrobial potential against pathogenic microorganisms isolated from currency (Naira notes).
Conclusion: AgNPs can be used as coating agents during currency production to minimize the spread of disease-causing pathogenic microorganisms.
Introduction
Currency notes are handled by large numbers of people, are widely exchanged for goods and services, or are presented as gifts. Currency notes are often contaminated by droplets during coughing, sneezing, touching hands, and placing on dirty surfaces during handling or transaction and thus are capable of absorbing, harboring, and transmitting infectious microorganisms1. In some countries, most people are not habituated to washing their hands after handling coins and banknotes. In some countries, many people are involved in the use of wetting fingers with saliva for manual counting of currency notes2. The salesmen or saleswomen of fish, poultry products, fruits, and vegetables in the markets or shops handle money and their respective goods simultaneously, neglecting hand washing between their tasks3. Banknotes are contaminated with oil, blood, and animal waste during food preparation or animal slaughtering4. Similarly, banknotes are stepped on by dirty shoe soles when they fall on the ground when sprayed during several events and ceremonies, and the continuous habit of squeezing paper currencies weakens them and makes them prone to microbial contamination. These unhygienic practices introduce the risk of cross-contamination, possibly resulting in random cases of infectious diseases among sellers and customers.
Paper currency notes are susceptible to bacterial contamination during continuous handling from person to person; they are stored in contaminated polythene and leather bags under moist, sweaty, and dark conditions that are favorable for the growth of different pathogenic microorganisms5, 6. The relatively dirt and older banknotes accumulate more microorganisms and increase the number of microorganisms circulating among their handlers, which indicates that the lower the index values of the money are, the greater the microbial contamination of the currency7. Paper currency money is a particular threat to public health since contagious diseases can broaden through contact with currencies8. The high rates of microbial contamination of currency by harmful pathogens could be associated with gastroenteritis, pneumonia, throat infection, tonsillitis, peptic ulcers, urinogenital tract infection, and lung abscess9.
Enterobacteriaceae, , , species, sp., sp. and sp. are likely contaminants of currency notes, which could be pathogenic, possess multidrug resistance and serve as a vehicle for infectious diseases in the community10. The contamination of pathogenic microorganisms on banknotes is of great concern for public health11. Currently, banknotes contain harmful microorganisms that are resistant to commonly used antibiotics, and contaminated currency notes remain in circulation without any discontinuity. Hence, there is a need for an alternative strategy to incorporate inhibitory substances into banknotes to suppress the incidence and occurrence of pathogenic microorganisms, which will minimize the transmission of diseases associated with pathogenic microorganisms. As a menace to curb the incidence of disease transmission through banknotes, nanocoating of currency notes is an eco-friendly approach for manufacturing new banknotes that prevents the growth of any pathogenic microorganisms and reduces cross-contamination and disinfection by reducing disease-causing microorganisms12. Nanoparticles (NPs) have been validated to be fascinating and effective at inhibiting infectious disease-causing microorganisms and multiple antibiotic-resistant bacteria. Therefore, in this study, silver nanoparticles (AgNPs) were biosynthesized via Ag reduction with the culture supernatant of isolated from Naira notes. The antimicrobial activity of -AgNPs (S-AgNPs) against multiple antibiotic-resistant microorganisms was established. This study further assessed the microbial quality of improvised currency notes coated with S-AgNPs.
Materials and Methods
Collection of Naira notes
Naira notes of different denominations were collected from the Akinjagunla market, Ondo city, Nigeria. Naira notes (15 each) of different denominations were obtained from shopkeepers, poultry product sellers, food vendors, and fish sellers.
Isolation and identification of microbial isolates from Naira notes
A wet sterile cotton swab was rubbed thoroughly on both surfaces of the naira notes. The swab was inserted into sterile peptone water. Serial dilutions were carried out, and an aliquot of 10 was spread on sterile nutrient agar and PDA (Hi-Media, India) plates. The plates were incubated at 37 °C for 24 hours for bacteria and at 25 °C for 48 hours for fungi. After incubation, distinct colonies were subcultured to obtain pure colonies. The colonies were Gram-stained and subjected to different biochemical tests, such as catalase test, coagulase, citrate, methyl-red, Voges-Proskaeur, and sugar fermentation, using the methods described by Olutiola and Cheesebrough14. Using the reactions of bacteria to biochemical tests, the identity of bacterial isolates was determined using Krieg .15.
Molecular identification of strain OS
The strain OS was selected for further studies because it can form silver nanoparticles with a change in color during the reaction. It was further identified using the 16S rRNA gene sequence-based method. The quality and integrity of the total genomic DNA of were checked by agarose gel electrophoresis and quantified by using an ultraviolet‒visible spectrophotometer (Shimadzu). PCR analysis was performed with a 16S primer. The PCR mixture comprised 50 ng of genomic DNA, 1.0 µL of 10X buffer, 0.4 µL of 50 mM MgCl, 0.5 µL of 2.5 μM dNTPs, 0.5 µL of 5 pmol of each primer, 1.25 units of 0.5 µL of Taq DNA polymerase and distilled water to make up a 10 µL reaction mixture. PCR was performed for 35 cycles in a Mycycler™ (Bio-Rad, USA) with initial denaturation for 3 min at 94 °C, cyclic denaturation for 30 s at 94 °C, annealing for 30 s at 58 °C and extension for 2 min at 72 °C with a final extension of 7 min at 72 °C. After PCR, the reaction products were analyzed by agarose gel electrophoresis.
The PCR products were purified with ExoSAP, and sequencing was performed using the Big Dye Terminator Cycle Sequencing Kit v.3.1 (Applied Bio Systems, USA). The sequence products were resolved on an Applied Biosystems 3130XL automated DNA sequencing system (Applied Biosystems, USA) at Macrogen, Inc., Seoul, Korea. The 16S rDNA sequence data obtained were further aligned using the BioEdit program. The 16S rDNA sequence of was analyzed by the Basic Local Alignment Search Tool (BLAST) bioinformatics program on the National Centre for Biotechnology Information (NCBI) website, and the sequences were compared with those in GenBank.
Biosynthesis of S-AgNPs
A pure culture of (18 h old) was inoculated into a 250 mL Erlenmeyer flask containing 100 mL of nutrient broth and incubated in an orbital shaker (BSOT-602, BioLAB, Canada) at 27 °C and 200 rpm for 48 hours. The cultured bacteria were harvested after 48 hours and centrifuged at 12,000 rpm for 15 min at 10 °C. The supernatant was collected for further reaction to synthesize nanoparticles16. The culture supernatant used for the production of silver nanoparticles was mixed with filter-sterilized AgNO solution at 1 mM. The bacterial biomass was taken for intracellular synthesis; 2.0 g of wet biomass was resuspended in 100 mL of a 1 mM aqueous solution of AgNO. The reaction between the culture supernatant and Agions was carried out under light conditions for 72 hours. Visual observation was conducted periodically to check for nanoparticle formation. The color change from yellow to brown indicated the production of silver nanoparticles (AgNPs) and the efficient reduction of Ag using
Characterizationof the biosynthesizedS-AgNPs
The formation of the reduced silver nanoparticles in the colloidal solution was monitored by using a UV‒vis spectrophotometer (Shimadzu). The absorption spectra were measured at 280-700 nm.
Antimicrobial Activity of S-AgNPs
Pure cultures of bacteria and fungi were subcultured on Muller–Hinton broth and potato dextrose broth, respectively, to obtain 18–24 h-old cultures. The turbidity of the inoculum was adjusted to 0.5 McFarland standard at 600 nm using a UV‒vis spectrophotometer (Shimadzu). The inoculum was spread uniformly on plates using sterile cotton swabs and allowed to stand for 40 minutes. Wells 4 mm in diameter were made on agar plates using a cork borer (4 mm). Using a micropipette, S-AgNPs at concentrations of 2.0 µg/mL and 5.0 µg/mL were dispensed into each well of a Petri dish. The experiment was performed in triplicate. The antibiotics amoxicillin and folic acid were used as positive controls. All the inoculated plates were incubated at 37 °C for 18-24 hours for bacteria and at 25 °C for 48-72 hours for fungi thereafter, the zone of inhibition was measured in millimeters (mm).
Circulation of the improvised coated Naira notes
The updated currency notes were coated with S-AgNPs by using a sterile brush and air-dried in a UV laminar hood (LHG-4AG, Germany). Three replicates of coated S-AgNP (60) and non-coated improvised currency notes (60) were distributed to different occupational groups, such as poultry product sellers (PSs), fish sellers (FSs), food vendors (FVs) and shop keepers (SKs). The improvised currency notes were allowed to thoroughly exchange hands and were collected for microbiological examination after 7 days.
Microbiological assessment of retrieved paper currency
Each of the improvised paper notes (coated with S-AgNPs and uncoated) was retrieved from various occupational groups. The improvised paper notes were soaked in 20 mL of sterile distilled water and vigorously agitated for 5-10 minutes. Serial dilutions of the resultant solution were made up to a factor of 10. One hundred microliters was pipetted into Petri dishes containing sterilized nutrients, and potato dextrose agar was added to the Petri dishes. The plates were incubated at 37 °C for 24 hours, while the PDA agar plates were incubated at 25 °C for 24 hours.
Data analysis
The data were statistically analyzed using SPSS version 20, the means of the zones of inhibition were separated using new Duncan’s multiple range tests and one-way ANOVA, and significant differences were considered at p≤ 0.05.
Results and Discussion
The bacterial and fungal counts (cfu/mL) from Nigeria currency collected from poultry products, fish, food and shopkeepers by different vendors are shown in
Microbial counts from Naira currency notes collected from shops and vendors
|
Weeks |
Poultry products’ sellers |
Fish sellers |
Food sellers |
Shop keepers |
Poultry products sellers |
Fish sellers |
Food sellers |
Shop keepers |
|
Bacterial count (106 × CFU/g) |
Fungi count (105 × CFU/g) | |||||||
|
1 |
7.5 |
4.7 |
5.9 |
2.7 |
3.0 |
2.2 |
2.0 |
1.6 |
|
2 |
7.8 |
5.9 |
6.7 |
4.5 |
4.7 |
1.2 |
3.2 |
0.3 |
|
3 |
5.9 |
3.3 |
7.9 |
2.6 |
1.2 |
2.7 |
1.6 |
0.6 |
|
4 |
2.8 |
6.7 |
3.0 |
1.4 |
4.0 |
2.9 |
1.5 |
2.4 |
|
5 |
3.5 |
4.8 |
4.5 |
3.0 |
1.8 |
2.2 |
3.7 |
0.6 |
|
6 |
3.8 |
4.7 |
3.8 |
2.8 |
2.5 |
4.3 |
1.8 |
1.0 |
The
Occurrence (%) of microorganisms in Naira notes from food vendors and shopkeepers
|
Microorganisms |
Poultry product seller |
Fish seller |
Food seller |
Shop keeper |
n |
% occurrence |
|
Bacteria | ||||||
|
S. aureus |
+ |
+ |
+ |
+ |
21 |
29.60 |
|
E. coli |
+ |
+ |
+ |
+ |
10 |
14.10 |
|
S. typhi |
+ |
+ |
+ |
+ |
8 |
11.30 |
|
P. aeruginosa |
+ |
+ |
+ |
+ |
8 |
11.30 |
|
S. pyogenes |
+ |
+ |
+ |
- |
6 |
8.50 |
|
B. subtilis |
+ |
+ |
- |
+ |
3 |
4.20 |
|
Fungi | ||||||
|
A. flavus |
+ |
+ |
+ |
+ |
8 |
11.30 |
|
R. stolonifera |
+ |
+ |
+ |
+ |
7 |
9.80 |
Fungal species were isolated from Naira notes. Ahmed .28 reported occurrence (3.12% — 25.70%) from currency notes collected from fish sellers, food vendors, poultry products sellers, and shopkeepers. Paper money was contaminated by several fungal pathogens, such as , , spp., spp., spp., spp., , , , spp., and spp., and parasitic species of different helminths, such as parasitic nematodes and tapeworms such as , , , hookworm, , and 26. The occurrence of microorganisms on banknotes remains an environmental vehicle for the transmission of potential disease-causing microorganisms8.
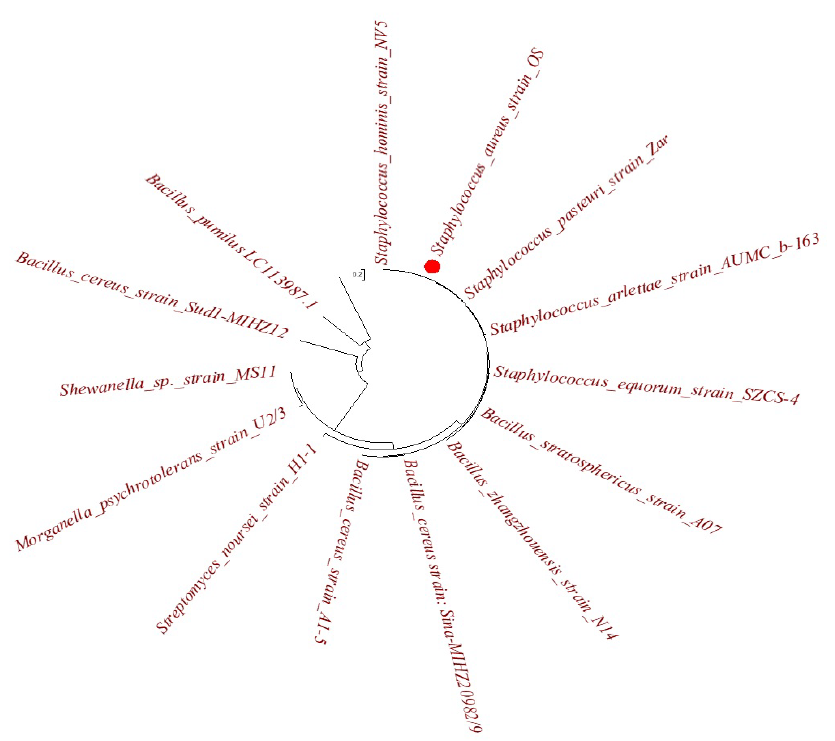
The molecular identity of the strain OS, a bacterium that reduces silver nanoparticles, is shown in Figure 1. The UV–Vis spectrum of the biosynthesized S-AgNPs exhibited absorption in the range of 300–700 nm, as shown in Figure 2. A peak at ~~420 nm was obtained for the S-AgNPs, which indicated the formation and stability of the reduced AgNPs. Nanda and Saravanan29 revealed a similar UV–Vis peak at approximately 420 nm, which confirmed the formation of metal nanoparticles with to provide surface plasmon resonance. UV‒Vis spectroscopy was used to detect the formation of silver nanoparticles when the colored nanoparticle solution showed a peak at ~400 nm30. The reduction of Ag ions was clearly visible when the supernatant of was added to AgNO, and the color changed from pale yellow to brown, indicating the formation of silver nanoparticles. The extracellular process involved in the reduction of metals, metal oxides, or metalloids for synthesis by microbial enzymes, proteins, and bacterial or fungal cell wall components is simple, convenient, inexpensive, stable, and eco-friendly31.

Evolutionary tree: the relationship between

UV‒Vis spectra of
S-AgNPs displayed zones of inhibition against all tested microorganisms (
Zones of inhibition (mm) by
|
Antibacterial agent |
Pseudomonas aeruginosa |
Bacillus subtilis |
Escherichia coli |
Salmonella typhi |
Streptococcus pyogenes |
A. flavus |
R. stolonifer | |||||||
|
2.0 µg/mL |
5.0 µg/mL |
2.0 µg/mL |
5.0 µg/mL |
2.0 µg/mL |
5.0 µg/mL |
2.0 µg/mL |
5.0 µg/mL |
2.0 µg/mL |
5.0 µg/mL |
2.0 µg/mL |
5.0 µg/mL |
2.0 µg/mL |
5.0 µg/mL | |
|
S-AgNPs |
7.70±0.08 e |
8.60 ±0.03 e |
12.60 ±0.31 c |
14.00 ±0.33 b |
8.8 ±0.00 e |
8.30 ± 0.17 e |
10.30 ± 0.10 d |
11.7 ± 0.01 c |
16. 00 ± 0.13 a |
17.30 ± 0.00 a |
12.10 ± 0.00 c |
14.7 ± 0.63 b |
12.00 ± 0.70 c |
17.30 ± 1.03 a |
|
Amoxicillin/Fulcine |
5.30 ± 0.00 d |
6.00 ± 0.00 d |
10.30 ± 0.31 b |
12.7 ± 0.11 a |
9.70 ±0.43 c |
8.3 ± 0.00 c |
10.00 ± 0.00 b |
11.6 ± 0.03 b |
10.00 ± 0.30 b |
11.3± 0.70 b |
10.00 ± 0.00 b |
13.33± 0.00 a |
11.00 ± 0.67 b |
12.70 ± 0.83 a |
The microbiological qualities of the improvised currency notes coated with S-AgNPs and noncoated currency paper are shown in
Microbial load obtained from customized currency notes coated with S-AgNPs and uncoated samples
|
Poultry-products Sellers |
Fish Sellers |
Food Vendors |
Shop Keepers | |||||
|
Bacterial count ×106 cfu/mL | ||||||||
|
Days |
A |
B |
A |
B |
A |
B |
A |
B |
|
1 |
0.0 |
3.20 |
0.0 |
2.00 |
0.0 |
1.60 |
0.0 |
3.20 |
|
2 |
0.0 |
4.00 |
0.0 |
2.30 |
0.0 |
3.50 |
0.0 |
3.30 |
|
3 |
0.0 |
2.00 |
0.0 |
2.70 |
0.0 |
6.00 |
0.0 |
2.50 |
|
4 |
0.0 |
8.00 |
0.0 |
1.02 |
0.0 |
1.00 |
0.0 |
1.20 |
|
5 |
0.0 |
2.20 |
0.0 |
1.55 |
0.0 |
1.10 |
0.0 |
1.00 |
|
Fungi count ×105 cfu/mL | ||||||||
|
1 |
0.0 |
7.00 |
0.0 |
3.40 |
0.0 |
5.90 |
0.0 |
4.20 |
|
2 |
0.0 |
2.00 |
0.0 |
1.30 |
0.0 |
1.20 |
0.0 |
1.14 |
|
3 |
0.0 |
1.03 |
0.0 |
1.10 |
0.0 |
1.40 |
0.0 |
1.30 |
|
4 |
0.0 |
1.02 |
0.0 |
7.00 |
0.0 |
1.06 |
0.0 |
1.02 |
|
5 |
0.0 |
2.05 |
0.0 |
1.80 |
0.0 |
2.50 |
0.0 |
2.30 |
Conclusion
S-AgNPs inhibited seven microorganisms isolated from Naira notes. Biosynthesized S-AgNPs can be coated on currency notes due to their potential antimicrobial activity against currency-contaminating microorganisms This will reduce the risk of infections associated with these pathogenic microorganisms present in banknotes. The hygienic status of banknotes needs to be improved by sensitizing the handling, vendor’s business owners, and individuals by government-approved agencies. Currencies can be coated with nanoparticles in an eco-friendly manner to prevent the risk of microbial infection on handlers.
Significance statement
The daily transactions of currency banknotes through many hands and contaminated counting machines with old age-currency notes have increased the transmission of disease-causing bacteria and fungi to humans. This, therefore, requires urgent attention. The use of nanoparticles in currency notes will suppress the growth of contaminated microorganisms and further minimize the transmission of diseases.
Authors' contributions
OOO and OSA contributed to the design of the study. OOO and OSA performed the experiments, while OOO and COO analyzed the data. OOO and COO prepared the manuscript. All the authors have read and approved the final manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest.

