The effect of reaction conditions and catalysts on the pyrolysis of polyethylene
- Department of Chemical Engineering, School of Chemical and Environmental Engineering, International University, Ho Chi Minh City, Vietnam
- Vietnam National University, Ho Chi Minh City, Vietnam
- NTT Hi-tech Institute, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam
- Hong Bang International University, Ho Chi Minh City, Vietnam
Abstract
Introduction: Pyrolysis of plastic is a green technology for converting plastic into fuel. This work studies the thermal and catalytic pyrolysis of polyethylene.
Methods: In this work, polyethylene was pyrolyzed in a batch reactor at temperatures ranging from 500 to 600 °C. Acid oxides (Al2O3 and TiO2) and base oxides (MgO and CaO) were used as catalysts. The catalysts were characterized by XRD to confirm the materials used.
Results: The results showed that the reaction temperature and reaction time affected the percentage of product fractions. A higher reaction temperature and time yielded a greater gas fraction, but hard conditions (600 °C and 3 h) produced a liquid fraction. The results also showed that thermal pyrolysis (for 3 h) yielded a greater liquid fraction and less gas fraction than that of catalytic pyrolysis (for 2 h and 3 h) at 600 °C. However, the use of oxide catalysts improved the quality of the liquid fraction compared with that of thermal pyrolysis, except for the TiO2 catalyst. Indeed, the results showed that the liquid oil fraction in catalytic pyrolysis exhibited a greater selectivity for hydrocarbons ranging from C7 to C20 than that in thermal pyrolysis. The base oxide catalysts (CaO and MgO) produced lighter hydrocarbons from C7 to C12, and the acid oxide catalyst (Al2O3) yielded a wider hydrocarbon distribution than that of the base oxide catalysts.
Conclusion: Pyrolysis is a promising method for converting polyethylene plastic into fuel. The quality of the liquid fraction was improved by using oxide catalysts for pyrolysis. The hydrocarbon range of the liquid fraction can be tailored by using different oxide catalysts with lighter hydrocarbons (C7 – C12) for CaO and MgO catalysts and a wider hydrocarbon range (C7 – C20) for Al2O3. The liquid fraction from polyethylene pyrolysis in this study can be used in gasoline and diesel fuel.
Introduction
Plastic, mostly originating from fossil fuel or petroleum, has various applications in many fields due to its practical and useful properties, such as flexibility, durability, high plasticity, and especially low production costs, making it a familiar material for every household and industrial field. Therefore, large quantities of plastics are produced each year to meet the high demand, dramatically increasing the amount of plastic waste. Global plastic production is estimated to be more than 400 million tons per year in 2021 and 2022, and global plastic waste generation is approximately 353 million tons per year 1, 2. However, only 15% of plastic waste is recycled2, 3. In Vietnam, approximately 7.5 million tons of virgin plastic pellets were imported, and ~ 2 million tons of virgin plastic pellets were domestically produced in 20234. Currently, the total output of the plastic industry is approximately 25 billion USD, exports in 2023 are approximately 4.5 billion USD, and plastic consumption in Vietnam has continuously grown by approximately 15% per year in recent years 4. Additionally, according to the Ministry of Natural Resources and Environment in 2023, Vietnam generates 1.8 million tons of plastic waste each year5. Vietnam is among the 20 countries with the largest amount of plastic waste, of which Hanoi and Ho Chi Minh City release approximately 80 tons of plastic waste into the environment every day 5. However, only 10% of plastic waste in Vietnam is recycled, and the remaining 90% of plastic waste is buried and burned or discharged directly into the environment5. This has several environmental and health implications, as plastic is slow to degrade, taking up to 400 years to decompose completely based on its chemical structure, and its derivatives are toxic.
Several outdated technologies, such as landfilling and incineration, have been employed to treat waste plastic. While they offer some benefits, they also have many critical disadvantages that can exacerbate the situation. For instance, landfills can cause air pollution and fould odors for nearby residents, as well as water pollution due to solid waste runoff. Incineration is a viable technology, but it can contribute to environmental pollution if not strictly controlled. Therefore, pyrolysis is considered a green technology worthy of study and development on a larger scale in the industry. Pyrolysis can convert plastic polymers into four fractions, solid, liquid, wax and gas, all of which have significant and useful properties as feedstocks for the petrochemical industry 6, 7.
Various catalysts have been utilized in the pyrolysis process, with zeolite8, 9, 10 being one of the most commonly used catalysts because zeolite is very good at cracking reactions for C–C scission reactions. However, zeolite is prone to coking due to its high acidity, and it has been reported to yield more gaseous products than liquid products 11, 12. Metal oxide catalysts are usually less acidic than zeolites and less expensive and more accessible, making them promising alternative catalysts for catalytic pyrolysis in the production of liquid products (10). Lopez et al. showed that red mud yielded a greater liquid fraction and lower gas yield in the catalytic pyrolysis of waste plastic than ZSM-5 at 500 °C (10). The hydrocarbon distribution of the liquid fraction obtained using the red mud catalyst ranged from C to C and was approximately 10% greater than that obtained using the ZSM-5 catalyst 10. AlO and ZnO oxides were used as catalysts in the pyrolysis of polyethylene plastic in a batch of Pyrex round-bottom glass13. Sierbet et al. 13 showed that AlO oxide produced a greater liquid fraction than did ZnO oxide, and both AlO and ZnO oxides supported the conversion of predominantly light hydrocarbons rather than heavy hydrocarbons, mostly ranging from C to C13.
Polyethylene (PE) is one of the largest plastics produced worldwide14. PE waste accounts for 40% of waste plastics 8 and can be converted to fuel, aromatics and light olefins by pyrolysis8, 15. Therefore, the conversion of waste PE into valuable products is an essential process. In this study, the thermal and catalytic pyrolysis of polyethylene were investigated to evaluate the effects of catalysts and reaction conditions on the conversion and product selectivity in the pyrolysis of polyethylene.
Materials and Methods
Materials
The polyethylene (PE) used in this study was low linear-low density polyethylene (LLDPE, JF19010, blown film grade, Reliance Polymers) and was obtained from Quy Thanh Wrapping print Production Co., Ltd., in Thu Duc City, Ho Chi Minh City. The catalysts used for this work were metal oxide catalysts, including the acid oxide catalysts AlO (Fisher) and TiO (Acros Organics) and the base oxide catalysts CaO (Xilong) and MgO (Xilong).
Characterization
The catalyst surface was analyzed by an X Shimadzu 6100 (Japan) instrument operating at a voltage of 40 kV and a current of 30 mA with CuK radiation at a wavelength of 0.15406 nm. The 2θ was set from 10 to 60, with each step being 0.02 and the scanning speed being 0.05/sec. The XRD peaks were compared with those of the standard JCPDS of all investigated oxides.
Thermal and catalytic pyrolysis
PE (5 g) was added to a batch reactor for thermal pyrolysis, and PE (5 g) was added with the catalyst (0.5 g)to the reactor for catalytic pyrolysis. The pyrolysis system contains two main parts: the reactor and condenser (Figure 1). The reactor and condenser were connected by a stainless-steel clamp. Before sealing the reactor and condenser, nitrogen was used to flush the air inside the reactor and condenser to ensure an inert atmosphere during the experiment. The condenser was cooled with ice water, and a balloon was connected to the top of the condenser to collect gas from the pyrolysis process. The heating furnace was heated to the desired temperature (500–600 °C, the most common temperature range used for plastic pyrolysis). After that, the reactor was placed into the heating furnace, and the experiment started. To finish the experiment, the reactor was removed from the heating furnace, dipped in ice water and kept for 25 minutes. The liquid and wax fractions in a warm reactor were separated from the solid fraction by slowly pouring the liquid fraction into a glass vial. The liquid fraction and wax fraction were separated after cooling at room temperature. The solid fraction was subsequently removed from the reactor by a spatula. All solid, liquid, and wax fractions were weighed by a digital balance, and then the mass of gas was calculated by using the initial mass of plastic to subtract the total mass of solid, liquid, and wax.
The yield for each product fraction is calculated by the following equations.
Yield of liquid oil (%) =
Yield of wax (%) =
Yield of solid (%) =
Yield of gas (%) =

The scheme of plastic pyrolysis.
The liquid fraction products were analyzed by an Agilent 6890 N gas chromatography (GC) coupled with an MSD 5973 mass spectrometer (MS). The column used was an Agilent HP-5MS 5% Phenyl Methyl Siloxane number Agilent 19091S-433 (30 m × 0.25 mm × 0.25 μm). The temperature of the inlet for mass spectrometry (MS) was 275 °C. The carrier gas was helium gas with a constant flow of 17.9 ml/min, a pressure of 1.70 psi, and a split ratio of 30:1. The total running time was approximately 32 minutes. The scanning mass range was from 35 amu to 650 amu, and the mass spectra were analyzed by the MSD ChemStation program with the NIST database.
Results
X-ray diffraction analysis
The XRD patterns of the investigated catalysts revealed that alumium oxide exhibited an amorphous structure with broad peaks (Figure 2), while TiO, CaO and MgO displayed crystallized structures with sharp peaks (Figure 2). MgO showed main peaks at 2θ values of ~ 43° and 62°, and CaO had main peaks at 2θ values of ~ 34° and 54°, consistent with the results of previous studies16 confirming the presence of MgO and CaO. In the case of TiO, the main peaks at 2θ values of ~ 25° and 48° indicate that TiO is in the anatase phase and aligns well with the standard JCPDS no. 84-1286 17.

XRD patterns of the investigated catalysts with crystallized structures of TiO2, CaO and MgO and amorphous structure of Al2O3.
Effect of temperature on thermal pyrolysis

Fractions obtained from thermal pyrolysis. Reaction conditions: 5 g polyethylene plastic, reaction time of 3 h. The liquid fraction was obtained at 600 °C.
Figure 3 shows the results of the thermal pyrolysis of polyethylene plastic in the batch reactor at 500 °C, 550 °C, 575 °C and 600 °C. The solid fraction was less than 16% in all cases, and it was a minority fraction compared with the other fractions. The wax fractions obtained at 500 °C, 550 °C, and 575 °C were 78.15 wt.%, 69.37 wt.%, and 60.91 wt.%, respectively. These waxes have a bad smell of burned polyethylene plastic. The color of the wax changed from white to yellow to light brown from 500 °C to 575 °C. The wax fraction remained in liquid form when the reactor remained hot, but upon cooling, it solidified into a soft solid at the bottom of the reactor, making it difficult to remove it from the reactor. The wax fraction tended to decrease with increasing temperature because a higher temperature provided more energy for cleaving the polyethylene plastic, resulting in the production of shorter hydrocarbon chain compounds, e.g., the gas fraction 18. This means that higher temperatures led to the rapid cracking of C–C bonds to form short chain hydrocarbons, e.g., gas. The yield of the gas fraction increased with increasing pyrolysis temperature. The yields of the gas fractions were 9.05 wt.%, 16.28 wt.%, and 24.51% at 500 °C, 550 °C, and 575 °C, respectively. However, no liquid was obtained at 500 – 575 °C after 3 hours. The high wax yield at 500–575 °C indicated that the polymer chain of the plastic had not partially cracked, and the hydrocarbon content of the wax still ranged from C to C19. When the temperature was increased to 600 °C, a liquid fraction of 44.31 wt% was obtained. The liquid emitted a faint smell of petrol and exhibited low viscosity. The liquid fraction had a dark red‒brown color and remained in liquid form even after the reactor had cooled. This suggested that a temperature of 600 °C was the proper temperature for the pyrolysis of polyethene in this study.
Effect of reaction time on thermal pyrolysis at 600 °C

Fractions obtained from the effect of reaction time on pyrolysis. Reaction conditions: 5 g polyethylene plastic and a reaction temperature of 600 °C. The liquid fraction was obtained at 600 °C and 3 h of reaction.
Figure 4 shows that the liquid oil fraction was observed during thermal pyrolysis at 600 °C for 3 h. Liquid oil was not obtained at lower pyrolysis times (1–2 h) (Figure 4). The wax yields were 59.47 wt.% and 55.57 wt.% after 1 h and 2 h, respectively. The yields of gas were 22.55 wt.% and 33.68 wt.% after 1 h and 2 h, respectively. The wax fraction tends to decrease, and the gas fraction increases with increasing reaction time. This trend was similar to that in a previous study on the pyrolysis of polyethylene in a batch reactor20. In fact, a longer reaction time will degrade heavy and light waxes into shorter hydrocarbon chains and liquids 20. In this study, for thermal pyrolysis at 600 °C, polyethylene, particularly in wax, possibly received enough energy to convert into liquid oil after 3 h of reaction. This suggests that reaction time is an important factor for cleaving the C–C bond of polyethylene to form different products.
Effect of AlO on the yield of pyrolysis

Fractions obtained from catalytic pyrolysis. Reaction conditions: 5 g polyethylene plastic, Al2O3 catalyst loading of 10%, temperature of 600 °C. The liquid fraction yield after 2 h of pyrolysis was slightly greater than that after 3 h of pyrolysis.
Notably, compared with thermal pyrolysis, the addition of AlO decreased the liquid fraction by 14.1 wt.% and increased the gas fraction to 4.41 wt.% (Figure 4 and Figure 5). The liquid fraction during the pyrolysis of polyethylene decreased due to the increase in the gas and solid fractions. However, the addition of AlO improved the quality of the liquid fraction. In the case of AlO oxide, the liquid fraction showed a clearer color than that of the liquid fraction from thermal pyrolysis. Additionally, the AlO oxide catalyst produced a petrol-like liquid, suggesting that the addition of AlO oxide enhanced the cleavage of polymer chains into short-chain hydrocarbon chains, which are in the range of gasoline fuel.
The liquid fraction was also obtained within 2 h of pyrolysis over the AlO catalyst, and the yield of the liquid fraction was not much different between 2 h and 3 h. Indeed, the liquid fraction yield after 2 h of pyrolysis was slightly greater than that after 3 h of pyrolysis. In addition, the liquid fraction after reacting for 2 h with the AlO oxide catalyst displayed a black and light green color, which was better than that of the liquid fraction after pyrolysis for 3 h (Figure 5).
For the solid fraction yield, the yield of the solid fraction when using the AlO oxide catalyst was approximately 20.86 wt.%, which was nearly double that of thermal pyrolysis. The solid fraction obtained over the AlO oxide catalyst was black, sticky and soft at the bottom of the reactor. These sticky soft solids even appeared in the liquid fraction and even floated on the liquid fraction. This phenomenon may be attributed to coke formation on AlO.
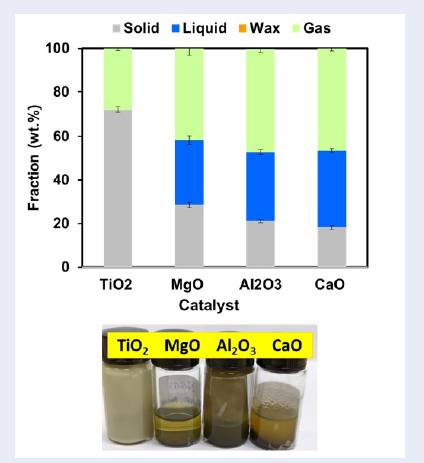
Comparison of acid and base oxide catalysts for catalytic pyrolysis
The production yield of thermal and catalytic pyrolysis of polyethylene at 600 °C and after 2 h of reaction
|
Catalyst |
Fraction (wt.%) | |||
|
|
Solid |
Wax |
Liquid |
Gas |
|
Thermal |
10.73 ± 0.24 |
55.58 ± 1.51 |
- |
33.69 ± 1.73 |
|
TiO2 |
72.17 ± 1.04 |
- |
- |
27.83 ± 1.04 |
|
Al2O3 |
21.15 ± 0.74 |
- |
31.68 ± 1.14 |
46.98 ± 1.88 |
|
MgO |
28.64 ± 1.18 |
- |
29.64 ± 1.95 |
41.72 ± 3.11 |
|
CaO |
18.25 ± 0.90 |
- |
35.08 ± 1.01 |
46.67 ± 1.25 |
It is obvious that TiO yielded a solid fraction without any liquid fraction, while CaO yielded the highest amount of the liquid fraction (Figure 6 and

Fractions obtained from catalytic pyrolysis. Reaction conditions: 5 g of polyethylene plastic, catalyst loading of 10%, temperature of 600 °C, and reaction time of 2 h. The liquid fraction was obtained over MgO, Al2O3 and CaO catalysts.
Discussion
The results of the pyrolysis of polyethylene indicated that the reaction temperature, reaction time and catalysts impact the process. An increase in pyrolysis temperature enhanced the formation of liquid but also produced more gas at higher temperatures. Additionally, a longer reaction time was necessary for the pyrolysis of polyethylene to produce liquid during subsequent pyrolysis. Interestingly, compared with thermal pyrolysis, the presence of oxide catalysts improved the quality of the liquid fraction. Nevertheless, the presence of oxide catalysts caused an increase in the solid fraction, which was due to the formation of coke. Coke formation can arise from the reaction mechanism of the pyrolysis process. The thermal pyrolysis of polyethylene involves several reaction mechanisms, including random scission, intermolecular hydrogen transfer, disproportionation, chain-mid β-scission and chain-end β-scission 21, 22. The presence of an acid catalyst (AlO) supported the cleavage of the C–C bond 23 to form a gas fraction. Both base oxides, MgO and CaO, also enhanced the formation of gas. Additionally, for coke formation, carbanions were formed in the pyrolysis of polyethylene and underwent cyclization to aromatics in the case of the MgO catalyst 24, 25, which can further convert to coke. The coke formation patterns observed in this study over AlO and CaO were similar to those observed in previous studies 26, 27. These results confirmed that the presence of both acidic and basic oxide catalysts favored the production of more gas and coke.
Regarding the yield of products, the liquid oil fraction obtained using catalysts in this study (~ 30 – 35%) was greater than that obtained in a recent study using Fe/ZSM-5 catalysts (21 – 28%) (
Summary of the thermal and catalytic pyrolysis of polyethylene
|
Seq. |
Sample |
Catalyst |
PE/cat. ratio |
Temp. (oC) |
Time (h) |
liquid oil (%) |
Solid residue (%) |
Gas (%) |
Ref. |
|
1 |
PE-460 |
- |
- |
460 |
3 |
86 |
1 |
13 |
|
|
2 |
PE-475 |
- |
- |
475 |
3 |
93 |
1 |
7 | |
|
3 |
PE-Z400-460 |
Y-zeolite |
10:1 |
400 |
3 |
37 |
2 |
32 | |
|
4 |
PE-Z410-430 |
Y-zeolite |
10:1 |
410 |
3 |
30 |
6 |
27 | |
|
5 |
PE-Z430 |
Y-zeolite |
10:1 |
430 |
3 |
49 |
34 |
18 | |
|
6 |
PE-Z430-5 |
Y-zeolite |
10:1 |
430 |
5 |
75 |
6 |
19 | |
|
7 |
PE-M4501-6 |
MgCO3 |
10:1 |
451 |
6 |
80 |
2 |
18 | |
|
8 |
PE-M4502-6 |
MgCO3 |
10:2 |
450 |
6 |
84 |
0 |
16 | |
|
9 |
HDPE |
Y-zeolite |
2:1 |
500 |
0.73 |
42 |
4 |
46 |
|
|
10 |
PE |
Al2O3 |
- |
500 |
Flow reactor |
76.46 |
7.62 |
15.92 |
|
|
11 |
LDPE |
ZSM-5 |
7:1 |
500 |
1 |
48 |
18 |
34 |
|
|
12 |
LDPE |
MgCO3 |
7:1 |
500 |
1 |
44 |
20 |
39 | |
|
13 |
LDPE |
Activated charcoal |
7:1 |
500 |
1 |
42 |
17 |
38 | |
|
14 |
PE |
5Fe/ZSM-5 |
- |
800 (1st stage) 500 (2nd stage) |
0.5 |
27.91 |
17.25 |
47.15 |
|
|
15 |
PE |
10Fe/ZSM-5 |
- |
24.16 |
24.32 |
46.81 | |||
|
16 |
PE |
20Fe/ZSM-5 |
- |
21.32 |
35.21 |
39.87 | |||
|
17 |
PE |
30Fe/ZSM-5 |
- |
23.98 |
29.82 |
41.61 | |||
|
18 |
LLDPE |
thermal |
- |
600 |
3 |
44.31 |
10.57 |
45.12 |
This study |
|
19 |
LLDPE |
Al2O3 |
10:1 |
600 |
2 |
31.68 |
21.15 |
46.98 |
This study |
|
20 |
LLDPE |
MgO |
10:1 |
600 |
2 |
29.64 |
28.64 |
41.72 |
This study |
|
21 |
LLDPE |
CaO |
10:1 |
600 |
2 |
35.08 |
18.25 |
46.67 |
This study |
Looking at the product selectivity of the liquid fraction (Figure 7 and

GC‒MS chromatograms of the liquid fraction after thermal and catalytic pyrolysis at 600 °C. The liquid fraction contains light hydrocarbons (C7 – C12, used as gasoline), middle hydrocarbons (C13 – C20, used as kerosene and diesel oil) and heavy hydrocarbons (C20+, used as lubricants and fuels).
The list of main hydrocarbons from GC‒MS results in the liquid fraction from thermal and catalytic pyrolysis of polyethylene
|
Entry |
Carbon number |
Chemical formula |
Chemical name |
|
1 |
C7 |
C7H14 |
1-Heptene |
|
C7H16 |
Heptane | ||
|
2 |
C8 |
C8H16 |
1-Octene |
|
C8H18 |
Hexane, 3-ethyl- | ||
|
3 |
C9 |
C9H18 |
1-Nonene cis-2-Nonene, |
|
C9H20 |
Nonane | ||
|
4 |
C10 |
C10H20 |
1-Decene cis-3-Decene |
|
C10H22 |
Decane | ||
|
5 |
C11 |
C11H22 |
5-Undecene, (E)- 3-Undecene, (Z)- |
|
C11H24 |
Undecane | ||
|
6 |
C12 |
C12H24 |
3-Dodecene, (Z)- |
|
C12H26 |
Dodecane | ||
|
7 |
C13 |
C13H26 |
1-Tridecene 5-Tridecene, (E)- 4-Nonene, 5-butyl- |
|
C13H28 |
Tridecane | ||
|
8 |
C14 |
C14H28 |
7-Tetradecene 3-Tetradecene, (E)- |
|
C14H30 |
Tetradecane | ||
|
9 |
C15 |
C15H30 |
1-Pentadecene |
|
C15H32 |
Pentadecane | ||
|
10 |
C16 |
C16H32 |
Cetene |
|
C16H34 |
Hexadecane | ||
|
11 |
C17 |
C17H34 |
8-Heptadecene |
|
C17H36 |
Heptadecane | ||
|
12 |
C18 |
C18H36 |
5-Octadecene, (E)- |
|
C18H38 |
Octadecane | ||
|
13 |
C19 |
C19H38 |
9-Nonadecene |
|
C19H40 |
Nonadecane | ||
|
14 |
C20 |
C20H40 |
1-Eicosene |
|
C20H42 |
Eicosane | ||
|
15 |
C21 |
C21H42 |
10-Heneicosene (c,t) |
|
C21H44 |
Heneicosane | ||
|
16 |
C27 |
C27H27 |
Heptacosane |
Conclusions
In this study, we investigated the effects of reaction temperature, reaction time and catalysts on the pyrolysis of polyethylene at 500–600 °C. The catalysts were characterized by XRD. We found that increasing temperature led to a decrease in the liquid or wax fraction and an increase in the gas fraction. The presence of oxide catalysts enhanced the quality of the liquid fraction derived from the pyrolysis of polyethylene plastic. The acid oxide catalyst, AlO, favored the production of light to middle hydrocarbons ranging from C–C. Base oxide catalysts such as MgO and CaO primarily enhanced the production of light hydrocarbons in the range of C to C. The liquid fraction was obtained with yields of 31.68%, 29.64% and 35.08% at 600 °C and after 2 h of reaction over AlO, MgO and CaO, respectively. Additionally, for different production purposes, each oxide catalyst can be used to produce a different range of hydrocarbons, which can be applied as gasoline or diesel fuel.
LIST OF ABBREVIATIONS
GC: Gas Chromatography
LLDPE: low linear-low density polyethylene
MS: mass spectrum
XRD: X-ray diffraction
COMPETING INTERESTS
The author(s) declare that they have no competing interests.
ACKNOWLEDGMENT
This work is funded by Hong Bang International University under grant code GVTC17.54.
Author contributions
Tung Thanh Tran: Writing – original draft, data curation and formal analysis
Huy Quoc Tran: Formal analysis, editing
Doan Hanh Tran: Formal analysis, editing
Thanh Ngoc Nguyen: Formal analysis
Thanh Khoa Phung: investigation, supervision, writing – review & editing
Tai Chiem Do: Funding acquisition, project administration, investigation, supervision, writing – review & editing

