Unlocking the Regenerative Potential of Antler Stem Cells: A Promising Frontier in Wound Healing and Tissue Repair
- VNUHCM-US Stem Cell Institute, University of Science Ho Chi Minh City, Viet Nam
Abstract
Stem cells have been playing an important role in regenerative medicine. Recently, deer antler stem cells have received more attention because of their unique regenerative abilities. Deer velvet is regenerated every year at a complete level and leaves absolutely no scars. It is a rare phenomenon in mammals at present. Thus, deer antler stem cells can be a potential agent of biomolecular, metabolic, and regenerative pathways. This review evaluated deer antler stem cells' wound healing and regenerative potential. Although mesenchymal stem cells have been studied extensively, studies on antler stem cells (ASCs) are still in their infancy. Due to ethical barriers, there is still few applications of ASCs in clinical. A more comprehensive understanding of deer antler stem cells will lay the groundwork for further studies to approach their applications in future.
Introduction
Current regenerative medicine advances have made much sense, as they are able to address most patients’ needs. However, regenerative medicine has always focused on "immortality", which includes the complete regeneration of lost tissues and organs, which is something that the current human race has not achieved. The regeneration mechanism, especially in higher animals, still needs to be fully understood. This requires additional financial investment and resources to explore. In the contemporary regenerative medicine landscape, researchers and clinicians are grappling with the complexity of orchestrating tissue regeneration across various biological systems. From skin wounds to intricate organ structures, the demand for effective regenerative solutions has never been more pressing. The limitations of current regenerative approaches, which are often hindered by scarring, fibrosis, and incomplete restoration of function, underscore the need for innovative avenues.
The four levels of regeneration in animals are classified as follows: 1) regeneration at homeostasis, replenishing and resisting daily wear, such as the epithelial layer or blood; and 2) self-healing or tissue regeneration, which occurs when the tissue is damaged to restore continuity. 3) Compensatory growth, increased cell size, increased cell division, or both to accommodate the functional load, which is commonly observed in the kidney. 4) Epigenetic remodeling, which is observed in . Mammals rarely have a fourth ability, but a unique case of deer antlers (Figure 1) can regenerate epigenetics.
In most mammals (including humans), fibrosis and scarring during wound regeneration are unavoidable1. However, observations of deer have shown that their pedicle wounds heal rapidly and do not leave any scars regardless of the wound area 2. Deer velvet has an annual growth cycle with a maximum growth rate of more than 2 cm per day 3. This development lasts approximately three months, with parts of this organ growing similarly, such as blood vessels, nerves, bones, and cartilage. Over time, the antler grows and becomes ossified to form deer antlers. The antler velvet is rich in macromolecules such as proteins and antioxidants, has very high concentrations of growth factors and is reduced by ossification4.

Structure of deer antlers
These features have attracted much attention from researchers worldwide, and Poland became the first country to generate a stable ASC line called MIC-15. Some studies have shown that ASCs can initiate the complete regeneration of antler velvet and promote perfect skin wound healing very quickly6, 7. ASCs are unique because they can renew the entire mammalian appendage (deer antler velvet). They are able to completely heal wounds without fibrosis and without scarring at the early stages of the velvet regeneration process each year. In addition, a number of studies have demonstrated that by transplanting ASCs into different models, wound regeneration also occurs. For example, research has shown that they have a very positive effect on a mouse model of injury6. In some other studies, the activity of ASCs was shown to be superior to that of human mesenchymal stem cells (MSCs) in preclinical trials6, 8. These findings demonstrate that small molecules such as miRNAs, polypeptides, and growth factors in ASCs have interspecies effects6.
ASCs exhibit unique properties, including nontumourigenicity, immunosuppression, scarless healing, antiaging attributes, and easy accessibility. Unlike MSCs, which have safety concerns related to tumorigenicity, ASCs lack tumor formation when implanted and exhibit controlled tumor growth in the context of rapid antler development. The immunomodulatory capabilities of MSCs, which are influenced by inflammatory cytokines, are paralleled by those of ASCs, which, despite being allogeneic, display low immunogenicity. Furthermore, ASCs exhibit immunosuppressive effects, making them promising options for stem cell therapy.9
Currently, there are no specific criteria for evaluating ASCs. Studies often rely on minimal criteria for evaluating human mesenchymal stem cells to confirm the ancestry of ASCs. ASCs have been shown to overexpress the markers CD73, CD90, CD105, NPM1, VIM, and Stro-1. In addition, ASCs also express several markers of embryonic stem cells, such as Oct4, SOX2, and Nanog; TERT CD9 and C-myc and Sox2, SSEA4, and Scripto-110, 11. Notably, there is an MSC population called multilineage-differentiating stress-enduring (Muse) (CD105+, SSEA3+) cells that also exhibit similar expression. This small subpopulation of mesenchymal stem cells has great potential and has only recently been of interest. Both of these genes are expressed at the same level as those in mesenchymal stem cells and are expressed at the same level as those in embryonic stem cells12.
Wound healing and tissue regeneration of ASCs
Wound healing and tissue regeneration
The process of wound healing intricately involves a myriad of diverse factors working in tandem, including cytokines, chemokines, cells, and conditions of the body. Typically, the wound healing process involves four (possibly overlapping) stages, as shown in Figure 2. When trauma occurs, the hemostasis phase is activated immediately. Its primary goal is to stop bleeding due to vascular disruption and fibrin clot formation. Moreover, 5–10 min of vasoconstriction is activated in the injured area 13. This quick response helps prevent further bleeding and protects the wound. When bleeding is controlled within the first 24 hours, neutrophils are delivered to the injured area and remain there for 2 to 5 days. These cell types, especially phagocytes, release mediators, such as reactive oxygen species (ROS), cytokines, and proteases14. These factors activate many pathways, and different types of cells are involved in this process. The removal of bacteria, cellular debris or foreign agents is the main goal in the inflammatory phase and helps to clean the wound. This activity is associated with the significant contribution of ROS overexpression. In addition, this phase creates the basis for the proliferation phase by releasing factors involved in cell growth pathways. For example, platelets typically release growth factors that stimulate the self-proliferation and migration of fibroblasts. Fibroblasts then synthesize extracellular factors, such as collagen, in the ECM to continue the process. This combination is simultaneous and continuous.
The proliferative phase occurs later, 5–7 days after the wound appears. At this stage, epithelialization and extracellular matrix remodeling help stabilize the wound. From the wound base, endothelial cells, fibroblasts, and other cell types are involved in this process—specifically, new blood vessel growth to deliver nutrients and oxygen to new tissue. The formation of substrates such as collagen, proteoglycans, and fibronectin14, 15 is temporary but essential for filling the wound, helping the wound shrink. In the final stage of this process, epithelial cells (fibroblasts, keratinocytes, etc.) regenerate on the wound surface. The regeneration of granulomatous tissue, including macrophages, fibroblasts, epithelial cells, and granulocytes, interweaved with capillaries and was based on temporary and loose collagen bundles. Many factors affect the rate of this process, such as wound moisture, collagen regeneration or intrinsic factors from the initial inflammatory process.14, 15 The remodeling phase lasts up to one year or more. During this phase, synthesis and degradation take place simultaneously. In adult wounds, Collagen 3 is replaced by Collagen 1, resulting in a more organized, fixed ECM of the wound with superior tensile strength compared with the original ECM. The peculiarity of this process is the appearance of myofibroblasts. ECM-degrading enzymes, especially those in the MMP family, contribute significantly to this process. Any disruption can lead to chronic wound formation14, 15. This process is summarized in Figure 2.

Wound healing involves four sequential but overlapping stages. These processes are interrelated, and the boundaries are not clear. (drawn with Biorender)
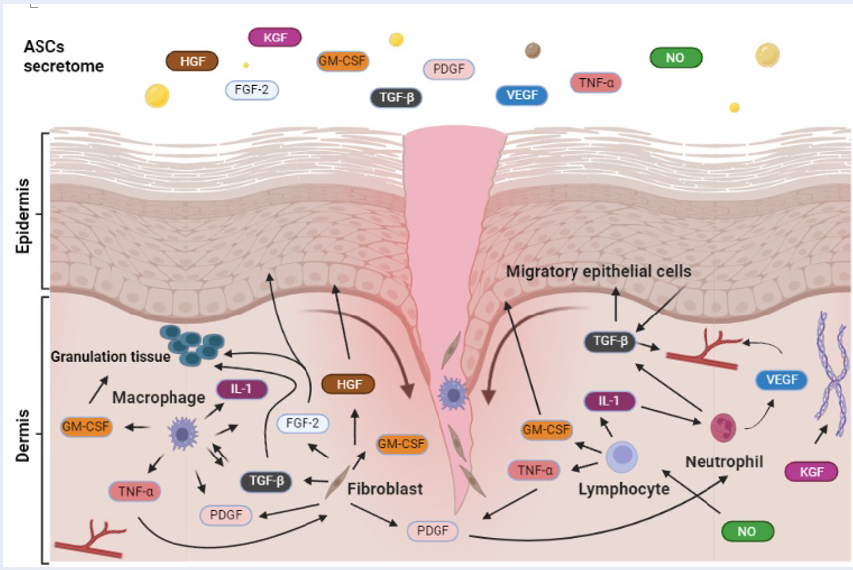
ASC secretory mechanism
Biomolecules of stem cells, such as cytokines, growth factors, or extracellular vesicles, play essential roles in tissue regeneration since they take part in the components of the tissue microenvironment. Similarly, ASCs also secrete factors that support the growth of cell populations and neighboring cells. Recently, active molecules secreted from ASCs have been confirmed (
Studies have demonstrated that deer antler cell factors are involved in wound healing.
|
Reference |
Growth factor |
Target cell |
Function |
|
Minkoo Seo et al Lai AK et al Jinghui Lei et al Cegielski et al[ |
FGF-2 |
Keratinocytes, Fibroblasts |
Re-epithelialization, stimulates angiogenesis and stroma, granulation tissue |
|
Nicole AM Minkoo Seo et al Cegielski et al |
IGF-1 |
Keratinocytes |
Stimulates proliferation and migration of keratinocytes |
|
Minkoo Seo et al Jinghui Lei et al Cegielski et al |
TGF-β |
Keratinocytes, Macrophages, Lymphocytes, Fibroblasts |
Re-epithelialization, stimulates angiogenesis and stroma, granulation tissue, keratinocytes migration and proliferation, inflammation |
|
Minkoo Seo et al Guo Q et al Kmiecik et al |
PDGF |
Keratinocytes, Endothelial cells, Fibroblasts |
Stimulates fibroblast proliferation, promotes matrix repair, granulation tissue, angiogenesis and wound contraction |
|
Minkoo Seo et al Lai AK et al Jinghui Lei et al Cegielski et al |
VEGF |
Keratinocytes, Endothelial cells, Fibroblasts |
Re-epithelialization, stimulates angiogenesis and stroma, granulation tissue, keratinocytes migration and proliferation, inflammation |
|
Minkoo Seo et al |
GM-CSF |
Epithelial Cells, Granulocyte, Macrophage |
Anti-inflammatory/regulatory cytokine |
|
Lin H et al |
NO |
lymphocytes |
Anti-inflammatory/regulatory cytokine |
|
Zhen et al |
HGF |
Epidermal cells |
Reepithelialization |
|
Cegielski et al |
NGF |
Keratinocytes, Fibroblasts and Mast cells |
Maintaining skin homeostasis |
|
Cegielski et al |
KGF |
Keratinocytes |
Stimulates collagen synthesis |
Cegielski et al (2013) first described the expression of many growth factors, such as IGF-1/2, TGF- 1, KGF, NGF, and BMP-2, in the ASC cell line MIC-1 38. High expression of VEGF in implanted ASCs supported blood vessel formation during hair growth in rabbits38. Later, via ELISA, Seo et al (2018) confirmed the presence of 26 secreted growth factors in ASC culture medium. PDGFs, VEGFs and TGF- 2 have been shown to be released mainly by ASCs39. The EGF level in ASC culture medium is significantly greater than that in MSC culture medium after 48 hours40. In mesenchymal stem cells (MSCs), PDGFs, VEGFs, TGF- , and EGF were shown to contribute to cell migration and support MSC tissue regeneration41, 42. Therefore, these secretomes from ASCs may play essential roles in better outcomes than those from human MSCs.
Effectively managing inflammation is crucial for promoting optimal wound healing. ASC-CM has the unique ability to augment anti-inflammatory macrophages, characterized by increased interleukin (IL)-10 and CD206 levels, while concurrently repressing proinflammatory macrophages (M1), which are responsible for TNF-α and nitric oxide synthase induction33. In vitro, ASC-CM significantly enhances LPS-stimulated RAW264.7 cell migration and modulates the expression of TNFα, IL-6, and IL-1β while coordinating the regulation of IL-10 and arginase 133. This study also demonstrated that nitric oxide (NO) from ASC-CM inhibited the pernicious effect of lymphocytes dispatched to the site of inflammation 33. Research by Chun et al. revealed a substantial surge in plasma IL-1β, IL-6, and TNFα levels in the untreated group, a surge effectively thwarted by the introduction of exosomes from ASCs. The treatment group displayed a notable increase in the level of IL-10, a pivotal player in suppressing proinflammatory cytokine production. This investigation underscores the efficacy of ASC-derived exosomes in alleviating postoperative cognitive dysfunction induced by cardiopulmonary bypass in mice, with the TLR2/TLR4 signaling pathway being implicated in this process43. The growth factor IGF-1 was proven to stimulate keratinocyte and fibroblast proliferation and to inhibit apoptosis during the wound healing process 23. Thus, it may play a role in reducing the production of inflammatory cytokines and promoting extracellular matrix production. This can be considered an indirect effect of ASCs related to wound immune regulation.
During the proliferation phase, the transformation of the transient wound substrate established during hemostasis into granuloma tissue is orchestrated. This burgeoning ensemble comprises an intricate interplay of fibroblasts, granulocytes, macrophages, blood vessels, and collagen bundles. Their collaborative efforts unfold like a biological symphony, not only restoring compromised structural integrity but also resuscitating the functional essence of the injured skin. This intricate process of cellular and molecular players serves as a regenerative overture, orchestrating the partial rejuvenation of the wounded terrain. Although preliminary, many studies have demonstrated that FGF-2, IGF-1, TGF-β, PDGF, HGF and VEGF (
The final phase of wound healing extends over a duration ranging from 21 days to 2 years (Figure 2)14. During this last and longest stage, collagen synthesis continuously strengthens the tissue. The process of regeneration unfolds as the wound undergoes ongoing contraction, leading to the reorganization of fibers 14. The appearance of the growth factors in

ASC secretions can promote wound healing, immune modulation and tissue repair. (drawn with Biorender)
Efficacy of ASCs and ASC-CM in animal experiments and clinical implications
Numerous studies have provided evidence supporting the safety and beneficial outcomes associated with the utilization of ASCs or ASC-CM. In a study conducted by Rong et al., the therapeutic effects and underlying mechanisms of ASC-CM on skin wound healing in mice were investigated. The findings revealed that ASC-CM notably promoted the proliferation of human umbilical vein endothelial cells and NIH-3T3 cells in in vitro experiments. Compared with that in the two control groups, the wound area in the group treated with ASC-CM was smaller. ASC-CM effectively accelerated wound healing and improved healing quality, possibly by converting wound skin fibroblasts into their fetal counterparts 6. The authors also demonstrated that the use of ASCs had a better therapeutic effect on radiation-induced skin damage than did the use of existing stem cells 53. Qianqian G and colleagues demonstrated that regenerative healing is not limited to a specific species and can be extended to other mammals. In their study, injections of ASCs facilitated the process of skin wound healing in mice24.
In a murine model of skin damage, treatment with ASCs resulted in faster wound healing and better regeneration. Owing to the presence of ASCs, mouse skin has the same structure of collagen fibers as nondamaged tissue. Conversely, the control group did not exhibit a comparable result, as collagen fibers were organized in parallel bundles characteristic of conventional scar tissue54. Another study by Lei et al demonstrated that exosomes from ASCs alleviate mesenchymal stem cell senescence and osteoarthritis. After treatment with exosomes from ASCs, the senescence phenotypes of human mesenchymal stem cells at late passages were significantly reduced, as demonstrated by improved proliferative capacity, an increased percentage of cells in S phase (synthetic phase) and an aging-related decrease in β-galactosidase activity. Minkoo Seo et al reported similar results 16. Truc LBP et al55 demonstrated that the inclusion of ASC extract in the foundation resulted in a notably stable serum product. Moreover, this serum significantly enhanced skin aging after only two weeks of consistent usage. ASCs can significantly reduce inflammation in gingival tissue by reducing osteoclast activation and inducing macrophage polarization toward the M2 phenotype. 33 Guokun Zhang et al56 demonstrated that antler peptides significantly reduced scar formation, accelerated wound healing, and improved wound quality. This involves stimulating the formation of new blood vessels (angiogenesis), increasing the quantity of skin appendages such as hair follicles and sebaceous glands, and enhancing the arrangement of collagen fibers in healthy tissue. Guokun Zhang et al[57 provided significant insights into the potential of ASC-exos in the process of regenerative wound healing, offering a promising method to reduce scarring and enhance wound healing quality. These findings illustrate that the effectiveness of ASC-exos not only matches that of ASCs but also surpasses that of exosomes derived from bone marrow mesenchymal stem cells in terms of potency. Particularly noteworthy is the positive impact of ASC-exos on the speed and quality of cutaneous tissue regeneration, including the restoration of hair follicles and sebaceous glands. These findings also indicate that ASC-exos have the ability to regulate the fibroblast-to-myofibroblast transition (FMT), a process often associated with scar formation. The ability of AnSC-exos to control FMT is crucial for maintaining the natural elasticity and quality of wound healing. These findings open up prospects for the use of AnSC-exos as a potential method to stimulate regenerative wound healing in a clinical setting, with the aim of reducing scarring and improving treatment outcomes57.
These effects are likely attributed to peptides derived from ASCs, as they demonstrated a notable ability to downregulate the expression of genes associated with pro-scar formation while concurrently upregulating the expression of genes related to anti-scar formation. Additionally, velvet antler peptides inhibit the TGF-β signaling pathway, resulting in a reduction in myofibroblast transdifferentiation and the formation of collagen I both in vitro and in vivo58. In general, the ability of ASCs to induce scarless wound healing processes in pathological animal models has led to the development of new therapies for clinical use.
A recent study demonstrated that ASC-conditioned medium exerted significant therapeutic effects in a streptozotocin (STZ)-induced model of type 1 diabetes (T1D) in mice. The results revealed that ASC-CM improved hyperglycemia, repaired pancreatic islet damage, and reduced liver dysfunction well beyond the effects of bone marrow stem cell-conditioned medium therapy. The main mechanism involved the extreme inhibition of the NF-κB pathway in the liver and pancreas. These findings suggest that AnSC-CM is a viable candidate for T1D treatment and associated liver complications59.
The incorporation of ASCs in therapeutic applications is impeded by significant ethical debates. Ethical considerations surround the potential side effects and risks inherent in the use of stem cells, and many of these risks remain inadequately addressed even when secretomes are employed as alternatives. Although the effects of ASCs on rabbit or mouse pathological models have been demonstrated to be better than those of human MSCs, there are still risks of infection and immune rejection, which are the greatest challenges in heterologous transplantation. To date, Marek Cegielski's study is the first study on the medical application of ASCS in humans 60. This study investigated the efficacy of ASC extract in a cohort of 20 dermatological patients with leg vein ulcers compared with a control group. Throughout the follow-up visits, measurements of the wound area and circumference were meticulously recorded. The findings indicated that, in addition to Ki-67 expression, all the examined parameters related to wound healing exhibited statistically more favorable outcomes in the experimental group. Despite the modest sample size, these promising results pave the way for the potential application of ASC therapies in diverse human diseases in the future.
Discussion
The utilization of animal-derived substances such as sheep placenta, honey, and deer antlers holds a significant position in traditional medicine, a practice rooted in healing methods that have spanned millennia. With their historical importance, natural products have been integral to therapeutic approaches for thousands of years. Deer antler velvet, in particular, has garnered interest because of its remarkable capacity for regeneration and swift growth (Figure 4). As a result, it has been found to have applications in medicinal and health food contexts.
In the current context, employing ASCs as cosmeceuticals has emerged as a fitting approach. The considerable potential of ASC secretomes in tissue engineering and regenerative medicine has been highlighted. These secretomes can instigate tissue repair, particularly in conditions involving skin defects. Recent research has underscored the therapeutic potential of EVs derived from stem cells. This avenue represents a strategic and promising application of ASCs in addressing various skin-related concerns61, 62, 63. There are three subtypes of extracellular vesicles distinguished by their biogenesis, release pathways, and functions: microvesicles, apoptotic bodies, and exosomes64. Exosomes excreted from ASCs contain 438 proteins that stimulate cell proliferation and migration or inhibit inflammation 18. These exosomes reduce aging in human stem cells and attenuate cartilage degeneration18. By inhibiting the TLR2/TLR4 signaling pathway, ASC-derived exosomes enhance cognitive function in cardiopulmonary bypass rats 65. Nevertheless, the production of secretions still lacks standardization despite extensive research on the pathways involved in their transportation. This finding indicates that while the transport mechanisms have been thoroughly investigated, achieving a standardized approach to secretion production remains an ongoing challenge in the field.

Number of studies on hydrogels subjected to burn treatment over time (Search ncbi, keyword: Antler stem cell).
In addition to their ability to promote skin wound healing, ASCs possess regenerative potential via other pathological mechanisms to restore tissue. They are capable of inducing bone regeneration and promoting the healing of critical-sized defects through osteogenesis and the modulation of local inflammation66, 52, 5. In cartilage repair, ASCs support remodeling of the matrix and chondrogenic differentiation, suggesting prospects in osteoarthritis and joint trauma67, 68. ASCs have also been shown to be efficacious in myocardial repair through paracrine-mediated angiogenesis and antiapoptotic effects. Liver injury models have shown improved hepatic function following ASC transplantation, which is attributed to their antifibrotic and immunosuppressive effects69. These findings affirm the multimodal mechanism by which ASCs can adapt to different types of tissue, highlighting their importance as therapeutic agents in situations other than cutaneous cases. Follow-up studies must aim at delineating the best delivery modality and long-term safety profiles with the goal of driving clinical translation.
The potential applications of ASCs are enormous. In the case of skin wounds, especially in elderly patients or diabetic open wounds59, some challenges require careful consideration when designing therapies. Abnormalities in tissues pose particular obstacles to adequate wound healing. The potential of ASCs presents a promising avenue for addressing wounds. The transition from foundational research to clinical studies is imperative, building upon recent advancements in understanding ASCs, which are continuously undergoing refinement for practical applications in the field of wound healing. However, there are still some restrictions on ASC utilization, such as variability in expansion and isolation processes, animal-to-human, and strict regulation of differentiation pathways to prevent unwanted lineage commitment. Additionally, tumorigenicity and immunogenicity issues, although negligible, have to be appropriately resolved by way of preclinical analysis to be able to provide biosafety for widespread clinical use.
Conclusion
The horizon for ASC products appears promising and has potential for effective applications in various realms, including tissue regenerative medicine, wound closure, tissue-engineered products, and the treatment of burn victims. As research and development progress, these ASC products are anticipated to play a pivotal role in advancing therapeutic interventions for a range of medical conditions, contributing to innovative and impactful approaches in healthcare.
Abbreviations
ASC: Antler stem cell, MSC, Mesenchymal stem cell, ROS, Reactive oxygen species, ECM, Extracellular matrix, PDGF: Platelet-derived growth factor, VEGF: vascular endothelial growth factor, MMPs: Matrix metalloproteinase, TIMP: Tissue inhibitors of metalloproteinases, HGF: hepatocyte growth factor, TGF-β: Transforming growth factor beta, TLR:Toll-like receptor
Acknowledgments
The authors sincerely thank Anh Le Tram Cao and An Phuc Huynh for their invaluable encouragement and unwavering support throughout the completion of this article.
Author contributions
Phat Duc Huynh developed the idea, painted and wrote the first draft, and collected the documents and data.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

