Rapid determination of magnesium and calcium ions in saline and seawater
- Faculty of Chemistry – University of Science HCMC
- Viet Nam National University, HCMC
- Faculty of Chemistry – University of Science HCMC Viet Nam National University, HCMC
Abstract
Access to fresh water from seawater treatment is crucial for sustaining human life. Furthermore, Ca and Mg in seawater represent valuable resources for agricultural and industrial purposes, warranting thorough analysis and recovery efforts. This study introduces the use of ortho-cresolphthalein complex one (o-CPC) for the rapid determination of Ca and Mg concentrations in saline and seawater samples. This approach utilizes a basic complexation reaction and follows the concept of a swift, point-of-care testing kit used to analyze samples, specifically biological specimens. The methodology was meticulously examined and optimized, encompassing key parameters such as reagent concentration, employment of masking agents, determination of optimal wavelengths, and assessments of accuracy and precision. The resulting standard curve exhibited high linearity (R2 > 0.995) across 0.5–5 mg. L−1 for Ca and 0.5–10 mg. L−1 for Mg. Notably, the method demonstrated good repeatability (% RSD < 5%) and achieved high recovery rates (95%–105%) when tested on spiked samples within real sample matrices. A comparative analysis between the o-CPC method and the F-AAS method (in accordance with the SMEWW 3111B:2017 standard) was conducted using three seawater samples, revealing no significant discrepancies in the analysis of Mg and Ca with a confidence level of 95%.
INTRODUCTION
The escalating issue of salinity presents a critical challenge that directly impacts the livelihoods of coastal communities, exacerbating freshwater scarcity. With its extensive 3,000 km coastline, Vietnam is directly affected by this challenge, notably in the Mekong Delta region, which is characterized by an intricate network of rivers bordering the sea. The dry season intensifies the complexity of saltwater intrusion, exacerbated by reduced upstream water flows, less rainfall (under 10 mm/month), and tidal inflows carrying seawater inland. Salinity levels surpass 45% along the Tien and Hau Rivers, peaking at 70% near river mouths and reaching 85% intrusion in certain areas. Consequently, ensuring a sustainable freshwater supply for irrigation and domestic usage requires ongoing research and development of desalination technologies 1.
Seawater predominantly comprises approximately 99.2% of ions, notably sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), chlorine (Cl), and sulfate (SO) ions. Na and Cl ions, which are the most abundant, are extensively utilized in saltwater treatment processes. Furthermore, brine contains economically valuable materials such as calcium carbonate and magnesium sulfate, making it viable for industrial and agricultural applications 2, 3. Calcium (Ca) and magnesium (Mg) are also essential trace elements present in seawater and exert a pivotal influence on establishing and regulating metabolic cycles in marine ecosystems. Additionally, the examination of magnesium and calcium provides a fundamental framework for evaluating diverse pertinent factors, particularly focusing on the desalination capabilities of various technologies. Consequently, there is a critical need for techniques to accurately determine the concentrations of these ions, especially in seawater or saline environments.
Various methodologies have been devised to evaluate the efficacy of desalination technologies by assessing the concentrations of Ca and Mg in seawater pre- and posttreatment. The titration method, outlined in the Vietnamese standard TCVN 6,201:1995 (ISO 6,059:1984), employs EDTA as a reagent with Erichrome Black T as an indicator. This method is widely utilized for the concentration analysis of these two ions in water owing to its simplicity. Nonetheless, the titration method suffers from limitations in accurately quantifying ions and is susceptible to interference from other metals that can form complexes with EDTA. As a result, measuring the levels of Ca and Mg in high-salinity water, which contains complex compositions of cations, is challenging. Alternatively, according to TCVN 6,201:1995, atomic absorption spectroscopy is commonly referenced, often involving the incorporation of lanthanum to eliminate interferences in specific sample matrices. Overall, this approach demonstrates a strong ability to selectively and effectively analyze the presence of Mg and Ca in seawater. Nevertheless, the abundance of salts in seawater can lead to the accumulation of salt deposits on the atomic absorption component, impeding the progress of the analysis procedure. Additionally, ion chromatography, specified in TCVN 6,660:2000 (ISO 14,911:1998), is utilized for analyzing Ca and Mg in brine solutions. Some drawbacks of this method include a restricted operational range, vulnerability to organic compound interference, and reliance on the lifespan of the chromatographic column. In pursuit of rapid and straightforward determination, this study adopts molecular absorption spectroscopy as the primary analytical method for assessing the Mg and Ca contents in seawater. The findings are juxtaposed with those obtained through alternative techniques.
Calcium and magnesium ions readily form complexes with various reagents, such as methymolblue, cagmagite, EDTA, and ortho-cresolphthalein complexone (o-CPC) 4. Among these methods, the colorimetric method employing o-CPC as a reagent is widely favored for quantifying Ca and Mg contents, especially in biological test kits commonly utilized in medical diagnostics, as corroborated by previous studies5. In this study, o-CPC (HZ) was chosen owing to its rapid and straightforward color development process, coupled with the stability of the resulting complex products, aligning with the objectives outlined in the study.
MATERIALS AND METHODS
Materials and instruments
Instrumentation
Absorbance measurements were conducted using a dual-beam spectrophotometer (Shimadzu Model 1800s) equipped with a 1-cm cuvette alongside a Perkin Elmer AAnalyst 700 flame atomic spectrophotometer.
Materials
Diethanolamine (>99%) was purchased from Xilong Scientific, China, and diethylamine (99%) from Scharlau, Spain, served as the buffer solvent. Stock standard solutions of Ca (1,000 mg. L) and Mg (1,000 mg. L) were supplied by Merck, Germany. New bottles containing these analytes were stored in a desiccator before use. o-CPC reagent (Fluka, USA) supplemented with 8-hydroxyquinoline (>99.5%) was chosen as the masking agent and was obtained from Shanghai Zhanyun, China. Distilled water was used throughout all the experimental procedures.
Reagent preparation
Preparation of the working standard solution involved diluting stock solutions of Ca and Mg, each at a concentration of 1,000 mg. L, is used to obtain solutions at 50 mg. L in distilled water.
To prepare 0.20 mM ortho-cresolphthalein complexone, 32.5 g of ortho-cresolphthalein complexone was dissolved in 250 mL of buffer solution at pH 11.5. This buffer solution was prepared using diethylamine at a concentration of 24 mM and diethanolamine at 0.5 M, with a ratio of diethylamine:diethanolamine:water = 1:20:400 (v/v).
To prepare 0.10 M 8-hydroxyquinoline, 750 mg of 8-hydroxyquinoline and 0.5 mL of sulfuric acid were dissolved in 50 mL of distilled water. The resulting solution was stored at 4°C in a dark vessel.
Sample preparation
Seawater samples were collected from three different locations in accordance with standards TCVN 6663-1:2011, TCVN 6663-3:2016, and TCVN 5,998:1995. These samples were stored under refrigeration at temperatures ranging from 1 to 5°C.
Subsequently, the samples underwent filtration and dilution with distilled water, employing an appropriate dilution factor.
General procedure
To determine the total concentration of Ca and Mg, 2 mL of the sample was added to a volumetric flask, followed by the addition of 3.5 mL of 0.2 mM o-CPC reagent to initiate the colorimetric reaction.
For Ca determination alone, 2 mL of the sample was added to a volumetric flask with 1 mL of 0.2 mM o-CPC reagent and 0.2 mL of 0.1 M 8-hydroxyquinoline masking agent to facilitate color development.
Upon the formation of the Ca–Mg–o-CPC complex, the absorbance of the solution was measured at the wavelength of maximum absorption (λ = 572 nm).
Optimization experiment
Under optimized conditions, the maximum absorption wavelengths for both the Ca–OCPC and Mg–OCPC complexes were determined by conducting a colorimetric reaction alongside an equivalent reagent blank sample across the 400–700 nm wavelength range. Sample concentrations of 3.00 mg. L for calcium Ca and 4.00 mg. L for magnesium Mg were employed in this investigation.
The calibration curve for the individual quantification of calcium and magnesium Ca and Mg ions was developed within concentration ranges of 0.5–5 and 0.5–10 mg, respectively. L, respectively. The experiments were conducted in triplicate.
The experiments were performed in a buffer environment with a pH of 11.5, focusing on the color reaction between Ca, Mg and o-CPC at various o-CPC concentrations ranging from 5 to 150 mg.L. The change in the absorbance of the resulting complex was measured through optical measurements at λ. Each cation was assessed at two concentration levels: 5 and 10 mg. L.
This assessment was conducted concurrently using a sample containing 2.00 mg. L Ca and a laboratory sample of a mixture with a Ca concentration maintained at 2.00 mg. L and a Mg concentration of 6.00 mg. L, with a Ca:Mg ratio of 1:3, representing the brine sample. The concentrations of 8-hydroxyquinoline investigated were prepared by weighing the appropriate amounts of reagent (ranging from 12.50 to 500.00 mg), dissolving them in 0.25 mL of concentrated HSO, and subsequently diluting them to 25.00 mL with distilled water.
The research sample solution was created by maintaining a constant volume of 1 mL of 100 mg. L Ca solution while varying the volume of 100 mg. L Mg solution within the range of 1–8 mL. The colorization process was performed following a methodology similar to that outlined in section 2.4, aimed at assessing the efficacy of the 8-hydroxyquinoline masking agent in eliminating interference.
The Con Dao seawater sample was selected to assess the repeatability of this method. The experiment was performed six times over three days. A 3% NaCl solution served as the blank. Standard solutions containing Ca and Mg at concentrations of 200 and 400 mg. L, respectively, were added. The accuracy of the method was evaluated based on the recovery rate.
The Ca and Mg contents in seawater samples were analyzed using the photometric method with the o-CPC coloring reagent and the comparative method F-AAS (following the SMEWW 3111B:2017 standard). A comparative assessment was conducted to analyze the outcomes of both approaches.
RESULTS
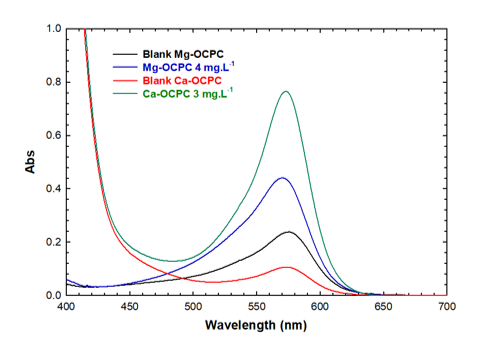
The UV–VIS absorption spectra of complex samples, including Ca–OCPC at 3.00 mg. L and Mg–OCPC at 4.00 mg. L, alongside their respective blank reagent samples, are depicted in Figure 1. Overlapping absorption peaks in the 500–600 nm region were observed.

UV–VIS absorption spectra of the CPC complexes and the blank. The black and red curves show the absorption of the blanks of Mg-OCPC and Ca-OCPC, respectively. The blue and green curves indicate the absorption of the complexes of Mg-OCPC and Ca-OCPC.
The repeatability and linearity were good, with an RSD <5% and a high correlation coefficient (R = 0.9989 and R = 0.9997) (Figure 2 and Figure 3).

Calibration curve for Ca using the o-CPC method with a concentration range of 0.5 – 5 mg. L-1. The error bar is standard deviations.

Calibration curve for Mg using the o-CPC method with a concentration range of 0.5 – 10 mg. L-1. The error bar is standard deviations.

Effect of the o-CPC reagent concentration on cation concentrations
The optical density of the solution was influenced by the reagent concentration. Figure 4 shows that as the reagent volume remains fixed, increasing its concentration leads to a corresponding increase in the optical absorption of the Mg–OCPC and Ca–OCP complexes. Depending on the sample’s Ca and Mg concentrations, the absorbance trends vary with the reagent concentration.
For Mg, at concentrations of 5 and 10 mg. L, no discernible difference was observed when reagent volumes were added at concentrations ranging from 5 to 50 mg.L. An optical density of 5 mg. L Mg solution remained relatively constant, while that of the 10 mg. L Mg solution exhibited increased optical density with increasing reagent concentration, up to 75 mg. L o-CPC.
A similar upward trend in absorbance was noted for Ca, with the critical reagent concentration for Ca being 5 mg. L and Ca 10 mg. L was determined to be 75 and 100 mg. L, respectively.
In this study, the influence of the 8-hydroxyquinoline masking agent concentration was examined. The data presented in Figure 5 indicate that in the absence of the masking agent, the optical absorbance of the sample solution increases. However, upon the addition of 8-hydroxyquinoline, the absorbance of the sample solutions gradually decreased. For this study, a recommended reagent concentration of 15,000 mg. L−1 was identified.

Effect of the magnesium 8-hydroxyquinoline masking agent concentration
The absorption of the solution was affected by the Mg:Ca ratio. As shown in Figure 6, the solution absorbance slightly decreased, while the optical density of the complex remained stable within the Mg:Ca ratio range of 1:1–3:1. A higher Mg:Ca ratio corresponded to reduced effectiveness of 8-hydroxyquinoline, leading to instability in the solution’s hue and rendering quantification unfeasible. Therefore, a recommended Mg:Ca ratio of 1:3 was identified.

Efficiency of 8-hydroxyquinoline in removing magnesium interference at different Mg:Ca ratios. The change in absorption of complexes within the Mg:Ca ratio range of 1:1-8:1 is displayed (the red curve). The error bar is standard deviations.
This method has good repeatability and %RSD values following AOAC regulations (
Results of the Con Dao seawater sample analyzed over 3 days
|
Cation |
Ca |
Mg | ||
|
Day |
C ± SD |
%RSD |
C ± SD |
%RSD |
|
Day 1 (n = 6) |
330 ± 13 |
3.9 |
1,300 ± 29 |
2.2 |
|
Day 2 (n = 6) |
311 ± 16 |
5.1 |
1,291 ± 43 |
3.3 |
|
Day 3 (n = 6) |
296 ± 14 |
4.7 |
1,248 ± 28 |
2.2 |
The recovery was within the specified range of the AOAC (95–105%) (
Recovery results
|
Recovery |
Average |
%RSD |
|
H% Ca |
102.0 |
3.2 |
|
H% Mg |
100.3 |
3.0 |
The analysis of seawater samples collected from three different sea areas, conducted using the o-CPC method, revealed consistent fluctuations in the Mg:Ca ratio, ranging between 3:1 and 4:1. This observed ratio aligns with established publications regarding ion concentrations in seawater. Specifically, the Ca concentrations in the seawater samples varied between 263 and 344 mg. L, while the Mg concentrations ranged from 1,014 to 1,345 mg.L. For the comparative method, Ca and Mg were analyzed by measuring the absorbance of the atom vapor at wavelengths of 422.7 and 285.2 nm, respectively, in the presence of La. The findings are presented in
Ca2+ and Mg2+ contents in the three seawater samples analyzed using the o-CPC and F-AAS methods.
|
Method |
Samples No. |
CCa ± SD |
CMg ± SD |
|
mg.L−1 | |||
|
o-CPC |
1 |
344 ± 16 |
1,339 ± 28 |
|
2 |
263 ± 5 |
1,014 ± 23 | |
|
3 |
310 ± 14 |
1,345 ± 22 | |
|
F-AAS |
1 |
342 ± 8.7 |
1,139 ± 29 |
|
2 |
260 ± 7.0 |
848 ± 28 | |
|
3 |
347 ± 9.0 |
1,255 ± 29 |
DISCUSSION
The results of the reagent investigation align with those of Cathryn M. Corns’ theory, which is based on the interaction between Ca, Mg and o-CPC 6. Notably, the reaction forms Mg–OCPC and Ca–OCPC in a molar ratio of 2:1, as depicted in Eqs. 1 and 2, showcasing the reaction processes.
The concentration of o-CPC, which ranged from 125 to 1,500 mg L, was stable, and the absorbance of the Mg–OCPC and Ca–OCPC complexes remained constant. Within this range, Ca and Mg in the solution fully reacted to form complexes with the reagent. Based on this observation, 130 mg Lwas identified as the optimal reagent concentration (Figure 4).
8-Hydroxyquinoline (8-HQ) was chosen as a preferred masking agent for Ca quantification alongside o-CPC owing to its diminished ability to form chelated complexes with Ca compared to o-CPC. Conversely, Mg exhibited a stronger chelating affinity for 8-HQ than for o-CPC (Figure 5).
The Mg:Ca ratio plays a crucial role in the color development process and the efficacy of 8-hydroxyquinoline in eliminating interference. Elevated ratios beyond 3:1 generated significant amounts of Mg-8HQ and released H, rendering the Ca–OCPC complex unstable7. Additionally, competition between the ligands o-CPC and 8-HQ for complexation with Ca ions was expected (Figure 6).
The standard curve model was utilized to quantify Ca and Mg using the o-CPC method, yielding satisfactory validation results. Comparisons of Ca and Mg contents in seawater samples determined through this method with those obtained via the F-AAS method exhibited favorable results. Statistical analyses, including the F test and t test, revealed no significant differences in variance values (F < F) or sample mean values (t < t) between the two methods for determining Ca and Mg concentrations in seawater, ensuring a reliability of P = 0.95. Particularly in the Ca analysis, there was notable consistency between the two methods based on the statistical analysis. It can be concluded that the o-CPC method is a reliable alternative for quantifying Ca and Mg in water.
Relative to the F-AAS method, the o-CPC method offered comparable precision (~2.0–5.0% vs. 2.6–4%) and high accuracy (95–105%) (Tables 1, 2).
CONCLUSIONS
In this study, the o-CPC method was successfully established and optimized, offering a rapid means of determining Ca and Mg due to two factors. The method is fast, involves an instantaneous colorimetric reaction, is simple and efficient, and can be adapted for kit-based testing formats.
In this study, the o-CPC method was successfully established and optimized, offering a rapid means of determining Ca and Mg due to two factors: the method exhibits high speed with an instantaneous colorimetric reaction, and the procedure is simple, efficient, and can be adapted for kit-based testing formats.
Notably, it is a straightforward and dependable technique for assessing Ca and Mg concentrations in complex matrices such as seawater or saline solutions.
Comparison with the reference method (F-AAS) demonstrated its suitability for analyzing standard samples exhibiting similar characteristics. The o-CPC method is promising for the rapid assessment of Ca and Mg contents in aqueous solutions and for efficiently evaluating the efficacy of water treatment technologies. Such applications represent valuable contributions to environmental treatment research.
ACKNOWLEDGMENTS
This research was funded by Vietnam National University Ho Chi Minh City (VNUHCM) under grant number B2023-18-05 to Truong Lam Son Hai.
AUTHOR CONTRIBUTION
Tran Duong Nhut and Tran Nhat Quang carried out the experiment with technical support from Hai Son Truong-Lam. Tran Nhat Quang wrote the manuscript in consultation with Hai Son Truong-Lam
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest related to this study.

