Solid dispersion system – An effective solution to enhance solubility of mangiferin
- Can Tho University of Medicine and Pharmacy
- School of Medicine, Vietnam National University Ho Chi Minh City
Abstract
Mangiferin - a xanthonoid abundantly present in Mango leaves has been widely recognized a potent hypoglycemic, antioxidant, and anti-inflammatory agent. Nevertheless, due to its poor solubility and poor permeability, its bioavailability and application in large scale remain limited. Amongst different methods for enhancement of such poor-soluble drugs, solide dispersion (SD) appears as a potent strategy, which is widely applied in pharmaceutical industry to enhance firstly dissolution rate and subsequently bioavalability of BCSII and BCSIV drugs. In this study, different solid dispersion (SD) systems of mangiferin with beta-cyclodextrin (β-CD), polyethylene glycol 6000 (PEG 6000), polyvinylpyrrolidone K30 (PVP K30), and hydroxypropyl methylcellulose (HPMC) 6M were prepared, using wet grinding and solvent evaporation methods, and compared in terms of mangiferin’s solubility. The results showed that the optimized SD of mangiferin and HPMC 6M (at 1:5 ratio), prepared by solvent evaporation method, achieved the highest dissolution rate of 81.96% after 30 minutes and 91.89% after 60 minutes at pH 1.2. Furthermore, differences in the material structure as well as the chemical composition between the optimized SD formula and raw Mangiferin were investigated and compared using the electronic microscopy (SEM), differential scanning calorimetry (DSC) and infra-red spectroscopy (IR). Overall, the findings within this study highlighted the potential of SD method in an attempt to enhance the solubility of active compounds in the class BCS II or BCS IV.
Background
Mangiferin is a C-glucosyl xanthone, which has been shown to have many pharmacological effects, such as antidiabetic, antitumor, lipometabolism regulating, antioxidant, analgesic, antibacterial, antiviral, and immunomodulatory ones 1. Therefore, Mangiferin is considered as a potential candidate for the treatment of several type of diseases, for instance, diabetes, cardiovascular diseases, etc. Nevertheless, due to its poor solubility and poor permeability, the bioavailabitity via oral administration of mangiferin is extremely low and this potential drug candidate belongs to group IV in the Biopharmaceutics Classification System (BCS)2. Therefore, there is a high demand to find out an effective solution to improve the solubility of mangiferin, which may faciliate the clinical application of this active compound. Amongst different available strategies to improve the solubility of a drug, including nanoformulation3, soya phospholipid-mangiferin complex 4, and solid dispersion 5, the Solid dispersion (SD) method appear as the most promising and applicable solution. Compared to other strategies, the SD technique presents several advantages, such as higher drug-carrying rate, simplicity, ease of implementation, and simple required equipment6. In this study, Mangiferin was firstly extracted from local mango leaves. Afterwards, different SD formula of obtained Mangiferin with different type of carrier and at different ratios were prepared and compared in terms of its solubility. Eventually, the optimal SD formulation of mangiferin was compared to the raw material in terms of material structure and chemical composition
Materialsand methods
Materials
Mangiferin was extracted from Cat-Chu Mango leaves (Mangifera indica L., Anacardiaceae) in Cao Lanh City, Dong Thap province, Vietnam. Thick mango leaves with dark green color,without pests, damage, termites were harvested in November 2022, washed with distilled water, and dried at 50-60 °C until unchanged weight, grinded and sieved to obtain coarse powder (According to Vietnam Pharmacopoeia V). Mangiferin was then extracted using 70% ethanol solution as solvent at a ratio of 1:15 (w/w), under sonication during 20 minutes. The extract was then evaporated at 60-70 C approximately 12 hours to obtain dry powder. The dry extract was dissolved in distilled water at the ratio of 1:2, extracted by ethyl acetate at the ratio of 1:1 (v/v), and the lower layer was taken and left for crystallization of mangiferin during 24 hours. The obtained suspension was filtered and dried at 50 C until obtaining a moisture content inferior to 5%. The purified mangiferin obtained was light yellow and odorless with a mangiferin content of 91.11%.
PEG 6000, β-CD, PVP K30, HPMC 6M, potassium chloride, distilled water, 96% ethanol and concentrated hydrochloric acid met pharmaceutical grade. Other chemicals and solvents were HPLC grade. Mangiferin reference had a CHOpurity of 97% calculated on an anhydrous basis was provided by the Institute of Drug Quality Control Ho Chi Minh City with the batch number of QT339 011020 and expiry date of 10/2023.
/-heart
Methods
Preparation of solid dispersion system to improve mangiferin solubility
Wet grinding (abbreviated as N) or solvent evaporation (abbreviated as DM) methods were used to prepare different solid dispersion of mangiferin and are detailed in
Formulas of solid dispersion system containing mangiferin
|
No. |
Formula |
Mgf: polymer |
Ratio (w/w) |
No. |
Formula |
Mgf: polymer |
Ratio (w/w) |
|
1 |
N1 |
Mgf: β-CD |
1:1 |
12 |
DM7 |
Mgf: PVP K30 |
1:5 |
|
2 |
N2 |
Mgf: β-CD |
1:2 |
13 |
DM8 |
Mgf: PVP K30 |
1:7 |
|
3 |
N3 |
Mgf: PEG 6000 |
1:3 |
14 |
DM9 |
Mgf: HPMC 6M |
1:1 |
|
4 |
N4 |
Mgf: PEG 6000 |
1:5 |
15 |
DM10 |
Mgf: HPMC 6M |
1:2 |
|
5 |
N5 |
Mgf: PEG 6000 |
1:7 |
16 |
DM11 |
Mgf: HPMC 6M |
1:5 |
|
6 |
DM1 |
Mgf: β-CD |
1:1 |
17 |
DM12 |
Mgf: PVP K30: β-CD |
1:5 (75:25) |
|
7 |
DM2 |
Mgf: β-CD |
1:2 |
18 |
DM13 |
Mgf: PVP K30: β-CD |
1:5 (90:10) |
|
8 |
DM3 |
Mgf: PEG 6000 |
1:3 |
19 |
DM14 |
Mgf: PVP K30: PEG 6000 |
1:5 (75:25) |
|
9 |
DM4 |
Mgf: PEG 6000 |
1:5 |
20 |
DM15 |
Mgf: PVP K30: PEG 6000 |
1:5 (90:10) |
|
10 |
DM5 |
Mgf: PEG 6000 |
1:7 |
21 |
DM16 |
Mgf: PVP K30: HPMC 6M |
1:5 (75:25) |
|
11 |
DM6 |
Mgf: PVP K30 |
1:3 |
22 |
DM17 |
Mgf: PVP K30: HPMC 6M |
1:5 (90:10) |
Wet grinding method: (1) Weighting of Mangiferin and carriers. (2) Mixture of Mangiferin and carriers. (3) Addition of sufficient amount of absolute ethanol to moisten the mixture powder, and then the mixture was ground for 30 minutes with a mortar and pestle to obtain a paste-like mixture. (4) Drying of the mixture at 50-60 ºC and stabilization in a desiccator for 24 hours. (5) Crushing and sieving through a 0.5 mm sieve. (6) Storage of the SD in an enclosed bottle in desiccator.
Solvent evaporation method:the steps (1), (4), (5), and (6) were similar to that of Wet grinding metho. (2) Mangiferin was dissolved in about 2/3 of ethanol:water (at the ratio of 2.5:1, v/v), then the mixture was stirred on a magnetic stirrer for about 30 minutes. Then, the carriers were dissolved into the above solvent mixture (3) The carriers were mixed with mangiferin, and then the mixture was stirred on a magnetic stirrer at 60ºC until the solvent was completely evaporated.
Dissolution test:Approximately 80 mg of solid dispersion containing mangiferin was taken into 500 mL of pH 1.2 medium. The solution was tested on a paddle-type dissolution tester with a rotation speed of 100 rpm and a temperature of 37 ± 0.5 ºC.
After 5, 15, 30, 45, and 60 minutes, 10 mL of the dissolved solution was taken and filtered. 1 mL of the filtrate was taken into a 10 mL volumetric flask, then pH 1.2 buffer was added to obtain a final volume of 10 mL. UV-VIS spectroscopy at 258 nm was used to quantity mangiferin7.
Blank sample: pH buffer 1.2. Each sample was analyzed in triplicate and the average was determined.
Uncorrected mangiferin concentration at the n time:
Cn and C were the corrected concentration and uncorrected concentration at the n time (µg/mL), respectively. C was the corrected concentration for the (n-1) time (µg/mL). V and V were volumes of dissolved solution and solvent (V = 10 mL and V = 500), respectively.
The percentage of dissolved mangiferin at the time t was calculated according to the formula:
n was the corrected concentration for the n time (µg/mL). m was the mangiferin content in the sample (mg).
Characterization of the solid dispersion system of mangiferin
Morphology
Scanning electron microscopy was used to study the surface of the obtained SD. Indeed, a thin layer of 2-3 mg powder was sprayed onto a metal disc with a piece of conductive double-sided tape attached. The sample was dried and then sprayed with an extremely thin layer of gold to avoid electrical charge on the surface. The specimen was put into the vacuum chamber and the surface of the particles was taken pictures by a scanning electron microscope (SEM, JSM – IT100 JOEL, 20 kV).
DSC analysis
Approximately 0.5 to 100 mg of sample was taken into a DSC aluminum dish on an analytical balance (a flat layer of solid with a height of 2 mm was the most appropriate) of NETZSCH: DSC 204 F1 PHOENIX appareil. It was covered by the lid sticking to the dish by force. To allow the air inside to expand, a small hole was drilled into the lid of the dish. A white aluminum dish was used as a comparison sample and the lid was punched with two holes to differentiate. The two aluminum dishes were put into the heating chamber with tongs. Temperature was increased from 30-250 C with a heating rate of 5 C per minute. The weight value was put into the computer and the results were processed.
IR analysis
About 1-2 mg of the sample was ground with 300-400 mg of KBr until homogeneous. Tablets with a diameter of 13 mm and a pressing force of 800 Mpa was fabricated using the obtained mixture and was IR analyzed, using IR Affinity 1S (Shimadzu) with a range of 4000–400 cm−1, resolution of 2 cm−1, and 128 scans.
Results
Preparation of solid dispersion ofmangiferin
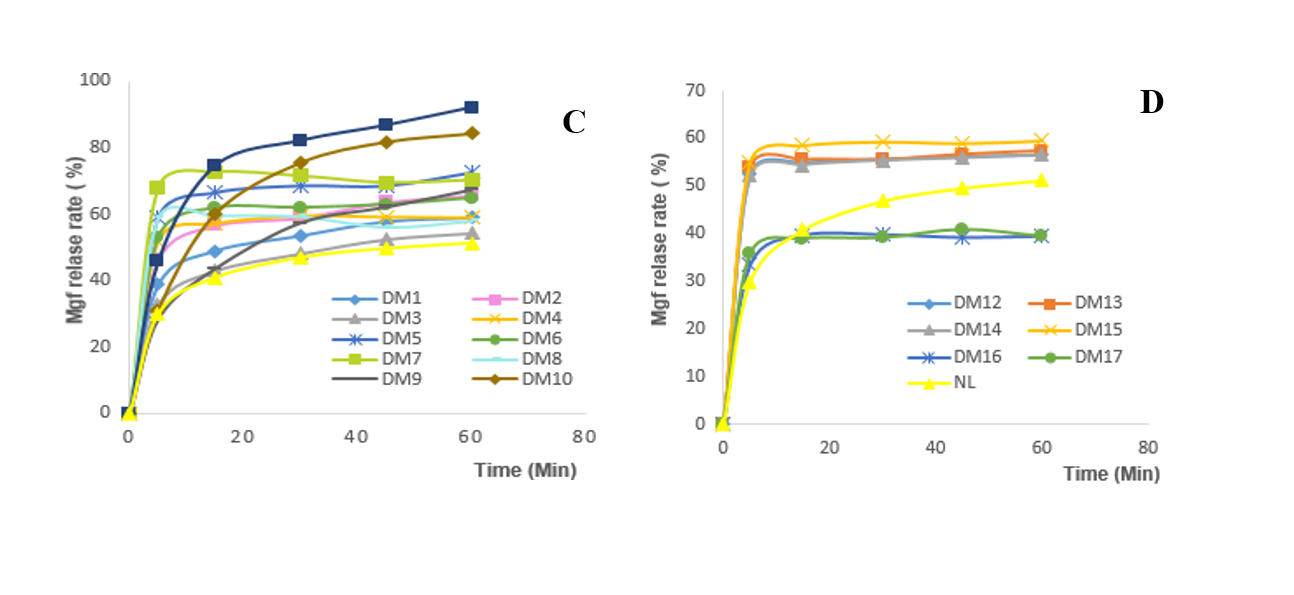
In total, eleven SD system of mangiferin with β-CD, PEG 6000, and PVP K30 at different ratios of 1:1, 1:2, 1:3, 1:5, and 1:7 (w/w) were prepared using wet grinding or solvent evaporation methods. Afterwards, the dissolution profile of pure mangiferin or SD mangiferin was determined and presented in Figure 1,

In-vitro release profiles of mangiferin solid dispersions prepared with the carrier of (A) pure mangiferin, (B) one carrier prepared by the wet grinding method, (C) one carrier prepared by the solvent evaporation method, and (D) two carriers prepared by the solvent evaporation method.
Value f2 similarity factor between solid dispersion system formulas
|
No. |
Formula |
Value f2 |
No. |
Formula |
Value f2 |
|
1 |
N1 |
35.15 ± 0.60 |
12 |
DM7 |
27.61 ± 1.96 |
|
2 |
N2 |
39.98 ± 1.16 |
13 |
DM8 |
38.22 ± 1.83 |
|
3 |
N3 |
30.23 ± 1.16 |
14 |
DM9 |
48.65 ± 0.90 |
|
4 |
N4 |
32.48 ± 0.91 |
15 |
DM10 |
29.14 ± 0.27 |
|
5 |
N5 |
32.48 ± 0.33 |
16 |
DM11 |
23.48 ± 0.97 |
|
6 |
DM1 |
54.24 ± 2.30 |
17 |
DM12 |
43.48 ± 0.79 |
|
7 |
DM2 |
41.37 ± 2.29 |
18 |
DM13 |
42.59 ± 0.61 |
|
8 |
DM3 |
76.47 ± 2.97 |
19 |
DM14 |
44.15 ± 0.46 |
|
9 |
DM4 |
41.43 ± 0.27 |
20 |
DM15 |
39.60 ±0.58 |
|
10 |
DM5 |
31.00 ± 1.44 |
21 |
DM16 |
55.39 ± 1.02 |
|
11 |
DM6 |
37.14 ± 1.15 |
22 |
DM17 |
55.41 ± 3.35 |
As showed in Figure 1, while the wet grinding technique showed a slight enhancement in dissolution rate of mangiferin (the highest dissolution rate was around 60% compared to 50%, respectively), the solvent evaporation method improved significantly the solubility of mangiferin (the highest dissolution rate was up to 90% compared to 60%, and 50%, respectively). In particular, the SD system DM11 made with HPMC 6M at a ratio of 1:5 (w/w) showed the highest solubility at approximately 90% with the lowest f of 23.48 ± 0.97 . To further confirm the result, the DM11 formula tested 6 times and the results were showed in
Mangiferin release rate of formula DM11.
|
No |
% Mangiferin release rate | ||||
|
5 minutes |
15 minutes |
30 minutes |
45 minutes |
60 minutes | |
|
1 |
45.71 |
74.39 |
81.96 |
86.57 |
91.89 |
|
2 |
44.92 |
74.03 |
81.67 |
85.84 |
91.77 |
|
3 |
45.63 |
74.95 |
82.23 |
85.96 |
91.88 |
|
4 |
45.74 |
74.54 |
81.77 |
86.51 |
91.82 |
|
5 |
45.69 |
74.43 |
81.89 |
86.61 |
91.75 |
|
6 |
45,56 |
74.27 |
81.91 |
86.49 |
91.87 |
Evaluation of the properties of the solid dispersion system
To clarify how the solid dispersion could change in the structure of mangiferin that lead to an increase in its solubility, three analytical techniques were applied, including IR, DSC, and SEM analysis. Indeed, when the solid dispersion system is formed, mangiferin is covered by hydrophillic careers, that leads to a significant increase of surface area, and results in a better solubility. Due to the covering of hydrophillic carrers, characteristic functional groups of mangiferin were obscured. Therefore, in this study, infrared spectroscopy was used to verify the presence or decrease in intensity of such functional groups. Due to the same reason, the endothermic peak of mangiferin itself and mangiferin + carrier was changed and the differential thermal scanning method was deployed to visualize thes changes. Finally, SEM images of mangiferin and the complexe mangiferin-carriers were recorded and compared in terms of the size and surface shape.
On the infrared spectrum of the solid dispersion system, a wavelength shift at the characteristic peak of the OH group from 3392 cm to 3444 cm was close to the peak of 3445 cm of HPMC. The pointed OH peak of the pure mangiferin was also replaced by a blunt peak, which indicated that the mangiferin was covered by HPMC. In addition, the peaks of 1253 cm-, 1221 cm-, and 1203 cm- were no longer seen, which were typical peaks for the CO group (Figure 2).

Infrared (IR) spectra of the pure mangiferin, the polymer HPMC, and the mangiferin solid dispersion with HPMC at a ratio of 1:5 w/w.
In DSC analysis (Figure 3), mangiferin had endothermic peaks at 106.4ºC, 142.0ºC, and 223.3ºC that was not observed in case of mangiferin SD. Furthermore, in SD system, the endothermic absorption peaks at 67.8 ºC and 211.9 ºC were similar to that of HPMC 6M at 70.1 ºC and 273.0 ºC, suggesting an interaction between mangiferin and HPMC in SD system.

Differential scanning calorimetry (DSC) graphs of the pure mangiferin (A), the polymer HPMC (B), and the mangiferin solid dispersion with HPMC at a ratio of 1:5 w/w (C).
SEM scanning image of solid dispersion showed that the surface of solid dispersion was similar to that of HPMC 6M, which also reconfirmed the previous findings that there existed an interaction between the active ingredient and the carriers (mangiferin was surrounded by HPMC 6M).

Scanning electron microscopy (SEM) images of the pure mangiferin (A, scale bar: 2 μm), the polymer HPMC (B, scale bar: 500 μm), and the mangiferin solid dispersion with HPMC 6M at a ratio of 1:5 w /w (C, scale bar: 2 μm).
Discussion
To improve the solubility of mangiferin using the Solid Dispersion (SD) technique, the first parameter to consider is the carrier. The current study investigated second-generation carriers including β-cyclodextrin, PEG 6000, PVP K30, HPMC 6M with a high level of biodegradability and biocompatibility and suitable for the solid dispersion technique 8. Among these potential carriers for the solid dispersion technique, β-cyclodextrin can form a soluble complex with mangiferin particles by providing an effective and hydrophilic shield, which also helps to prevent the recrystallization of the drug and keep the drug in a stable amorphous form. Both wet and solvent grinding methods resulted in a good solubility improvement at a mangiferin:β-cyclodextrin ratio of 1:2. With the carrier PEG 6000 and PVP K30, which are polymers with linear chains and soluble in water, PEG 6000 and PVP K30 can create intramolecular hydrogen bonds with mangiferin particles, forming a complex that makes mangiferin exist in an amorphous state. The results show that PVP K30 with a ratio of 1:5 has higher solubility than a ratio of 1:7, while PEG 6000 with a ratio of 1:7 has the highest solubility. This difference may be due to an increase in viscosity of the formed solution. Indeed, in the case of PVP K30, the higher the usage rate is, the higher the viscosity, which can hinder the dissolution process. In contrast, as PEG 6000 has many -OH groups, the effect of increasing solubility is overwhelming its viscosity-inducing effect.
A combination of two carriers within an SD system was proven not to help improve solubility significantly but may be hindered due to occasional interaction between polymers that lead to changes in their properties9. In this study, HPMC was shown to be the most appropriate carrier for the mangiferin SD system with a dissolution rate of 81.96 ± 0.05% after 30 minutes (1.75 times higher than pure mangiferin at the same time) and 91.89 ± 0.38% after 60 minutes (1.80 times more than pure mangiferin at the same time). This benefit may be explained by the viscosity properties of HPMC 6M, which helps in preventing the crystallization of large particles of mangiferin during the formation of the solid dispersion system. In addition to the choice of carrier, the ratio of active ingredients to carriers is also an important factor that significantly affects drug solubility. In this study, a ratio of 1:5 between drug and carrier was found to be optimal, which is also commonly used in the literature for SD technique5. Moreover, increasing the ratio up to 1:7 was practically impossible due to elevated viscosity. Besides, when using a large amount of HPMC, a polymer-rich diffusion layer may be created around the active ingredient, inhibiting the diffusion of the active substance into the environment during dissolution10.
The method used to fabricate the SD system also had an important impact on the obtained solubility. As confirmed in the current study, the solvent evaporation method was more appropriate for the mangiferin SD system. At first sight, this method is highly recommended as it is simple and easy to scale up. Furthermore, many studies in the literature have also demonstrated that the solubility of active substances can be better enhanced using solvent evaporation than by other methods 11.
In terms of characterization of the mangiferin SD system, as expected, all IR, DSC, and SEM analyses proved there was an interaction between mangiferin and its carrier. Indeed, the poorly water-soluble mangiferin was covered by a hydrophilic polymeric layer that explains also an important enhancement of its solubility. This interaction, as well as the covering of active ingredient by the polymeric layer in the SD system, was also observed in literature, such as the study by Mai Hoang Anh et al., 12.
Conclusion
In this study, an optimal solid dispersion system of mangiferin and HPMC 6M at a ratio of 1:5 was successfully formulated and fabricated using the solvent evaporation method, which was 1.8 times higher than that of pure mangiferin. For all obtained results, the current study highlighted the potential of the SD technique in firstly improving the solubility and subsequently the bioavailability of drugs in BCS II and BCS IV, as an attempt to make such potent compounds more applicable in drug development.
Abbreviations
xxx
Acknowledgments
xxx
Author’s contributions
All authors read and approved the final manuscript.
Funding
xxx
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

